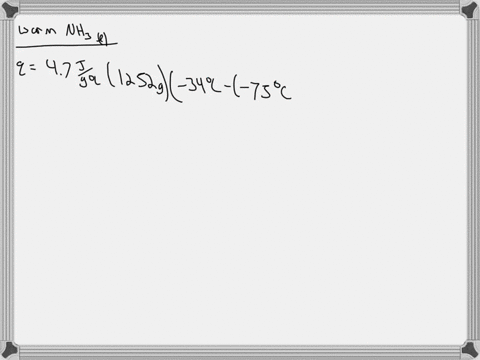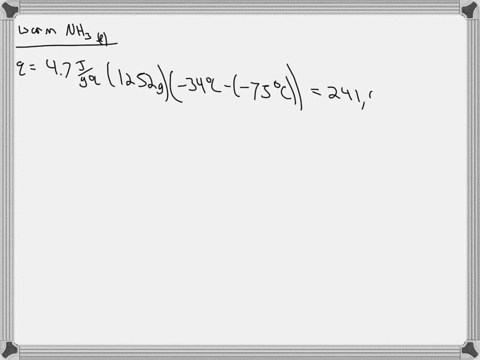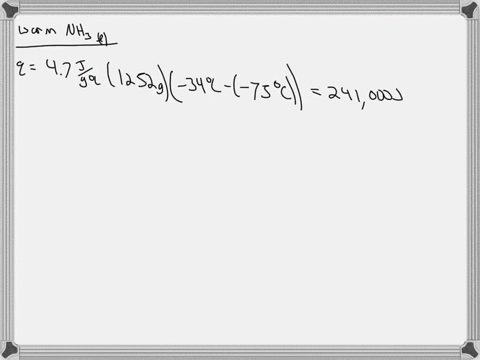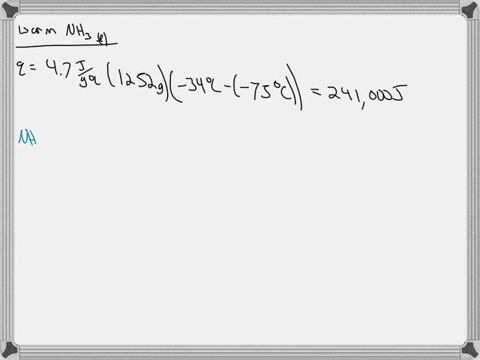Question
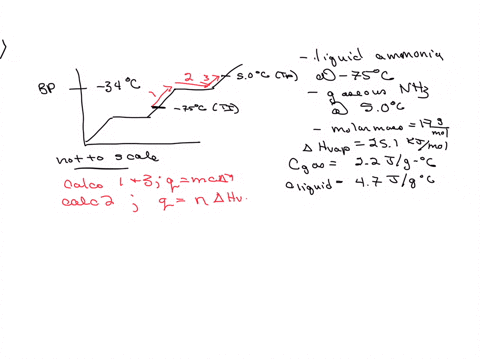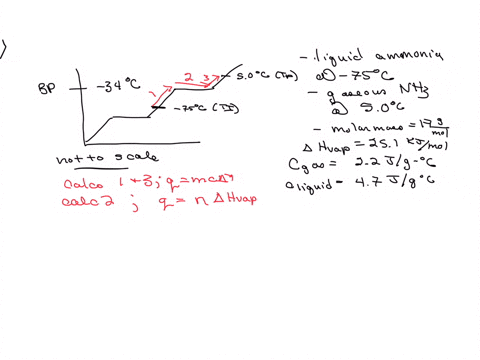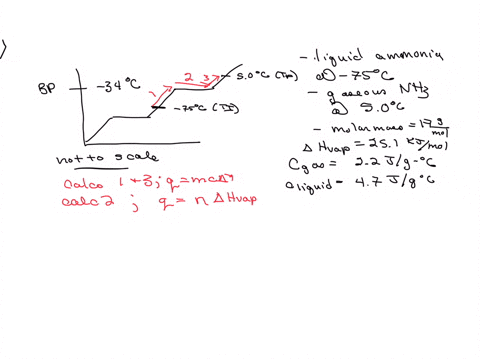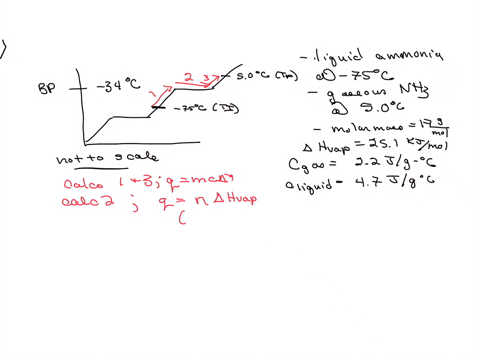
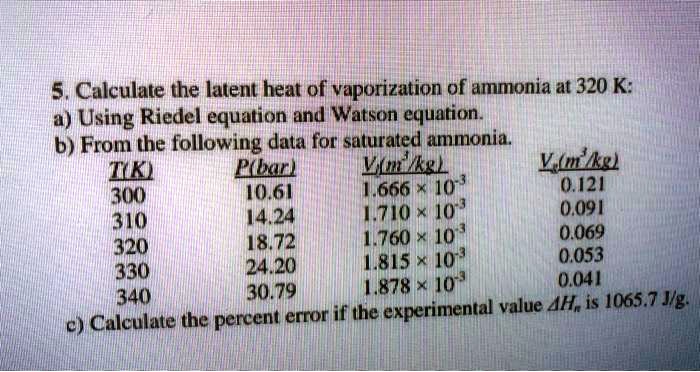
5. Calculate the latent heat of vaporization of ammonia at 320 K: a) Using Riedel equation and Watson equation 6) From the following data for saturated ammonia TK Ebanl VLkL Vlukgl 300 10.61 666 * To ' 0.121 14.24 1710 * 10 ' 0.091 310 18.72 760 101 0.069 320 24.20 1815 10^3 0.053 330 1878 1073 0.041 340 130.79 experimental value AH; is 1065.7 J/g: Calculate the percent error if the C)
5. Calculate the latent heat of vaporization of ammonia at 320 K: a) Using Riedel equation and Watson equation 6) From the following data for saturated ammonia TK Ebanl VLkL Vlukgl 300 10.61 666 * To ' 0.121 14.24 1710 * 10 ' 0.091 310 18.72 760 101 0.069 320 24.20 1815 10^3 0.053 330 1878 1073 0.041 340 130.79 experimental value AH; is 1065.7 J/g: Calculate the percent error if the C)
Show more…
Added by William W.
Instant Answer
Step 1
We need to find ΔHvap at 320 K. Let's assume T0 = 273 K (standard reference temperature). From the given data, we can calculate the vapor pressures at 300 K, 310 K, 320 K, 330 K, and 340 K using the Clausius-Clapeyron equation: ln(P/P0) = -ΔHvap/R * (1/T - Show more…
Show all steps