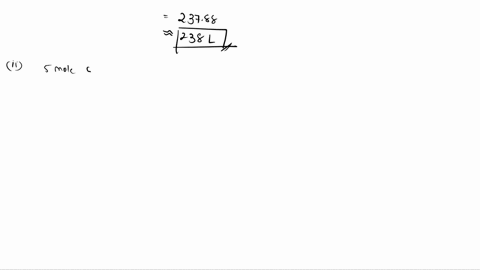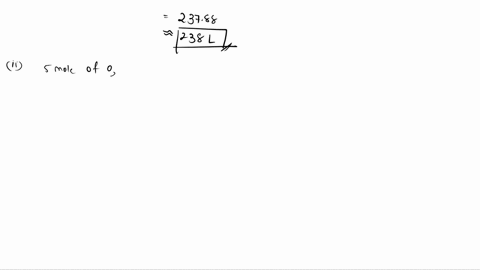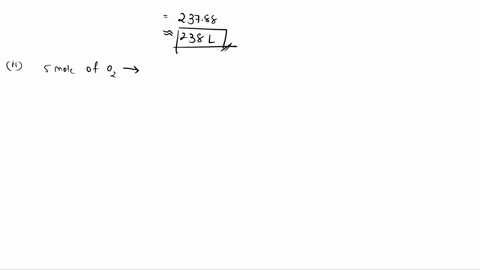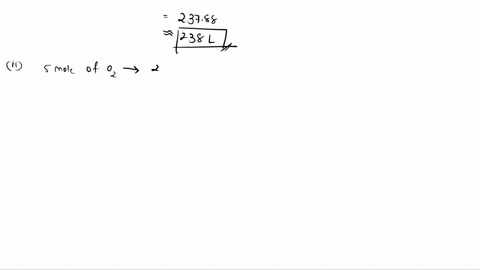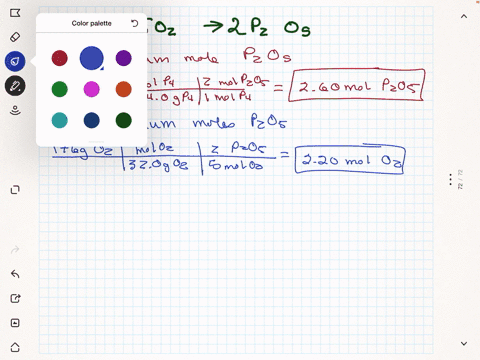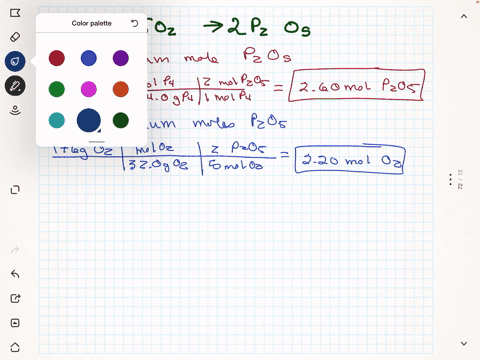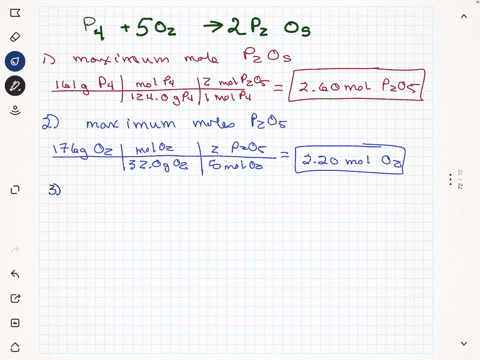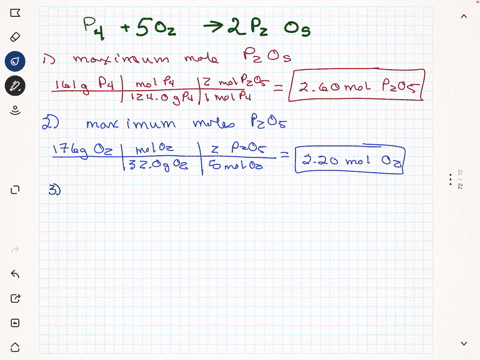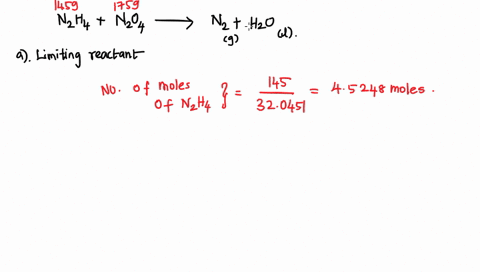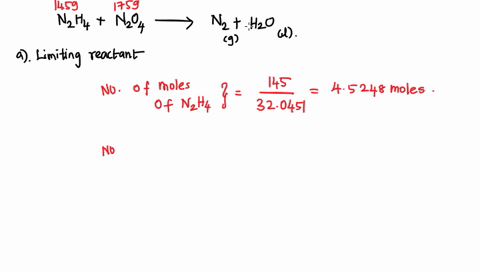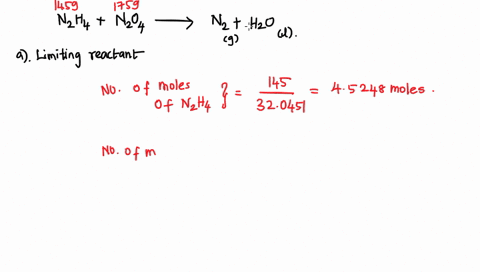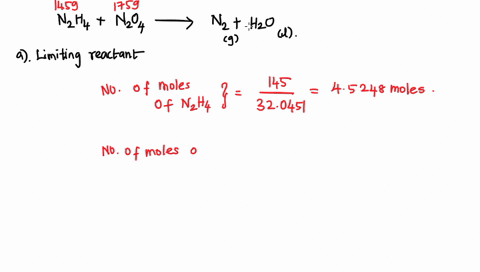Question
6. (This question is related to questions 4 and 5 seen below) Elemental phosphorus is produced by the reaction, 2 Ca3(PO4)2 + 6 SiO2 + 10 C ? 6 CaSiO3 + 10 CO + P4 Suppose that you have 9.0 moles of Ca3(PO4)2, 21.0 moles of SiO2, and 49.0 moles of C. (a) Which reactant is limiting? (b) What are the maximum amounts (in moles) of CaSiO3, CO, and P4 that can be produced? ------- 4. You are making double-decker sandwiches; each sandwich consists of 3 slices of bread, 2 chicken slices, and 2 tomato slices. Suppose that you have 84 slices of bread, 64 chicken slices, and 58 tomato slices. (a) What is the maximum number of complete sandwiches you can make with the quantities you have available? (b) Which ingredient is the limiting one? (Which one do you run out of first?) 5. The figure on the next page represents a reaction mixture consisting of gaseous N2 and H2 which react according to the following equation: 3 H2 + N2 ? 2 NH3 (a) What is the maximum number of product molecules that can be formed from the quantities available? (b) What is the limiting reactant? (c) How many molecules of the excess reactant remain when reaction is complete?
6. (This question is related to questions 4 and 5 seen below)
Elemental phosphorus is produced by the reaction, 2 Ca3(PO4)2 + 6
SiO2 + 10 C ? 6 CaSiO3 + 10 CO + P4 Suppose that you have 9.0 moles
of Ca3(PO4)2, 21.0 moles of SiO2, and 49.0 moles of C.
(a) Which reactant is limiting?
(b) What are the maximum amounts (in moles) of CaSiO3, CO, and
P4 that can be produced?
-------
4. You are making double-decker sandwiches; each sandwich
consists of 3 slices of bread, 2 chicken slices, and 2 tomato
slices. Suppose that you have 84 slices of bread, 64 chicken
slices, and 58 tomato slices.
(a) What is the maximum number of complete sandwiches you can
make with the quantities you have available?
(b) Which ingredient is the limiting one? (Which one do you run
out of first?)
5. The figure on the next page represents a reaction mixture
consisting of gaseous N2 and H2 which react according to the
following equation: 3 H2 + N2 ? 2 NH3
(a) What is the maximum number of product molecules that can be
formed from the quantities available?
(b) What is the limiting reactant?
(c) How many molecules of the excess reactant remain when
reaction is complete?
Show more…
Added by Austin G.
Instant Answer
Step 1
To do this, we will compare the mole ratios of the reactants to the balanced chemical equation. The balanced chemical equation is: 2 Ca3(PO4)2 + 6 SiO2 + 10 C → 6 CaSiO3 + 10 CO + P4 We have 9.0 moles of Ca3(PO4)2, 21.0 moles of SiO2, and 49.0 moles of C. For Show more…
Show all steps