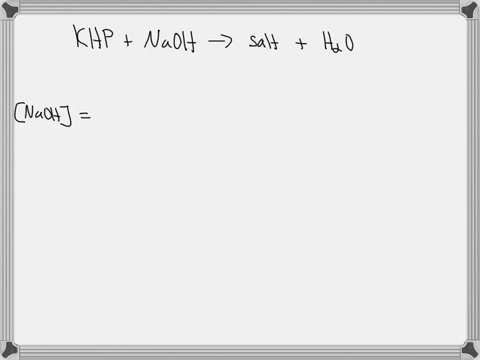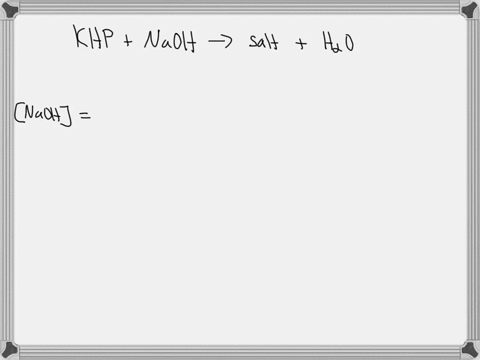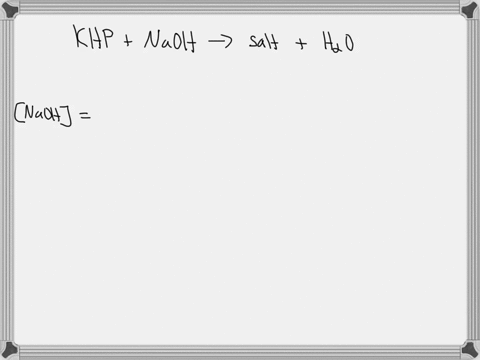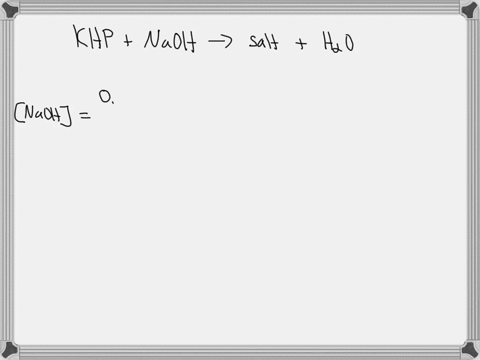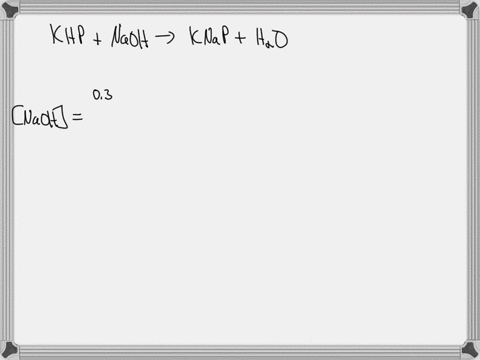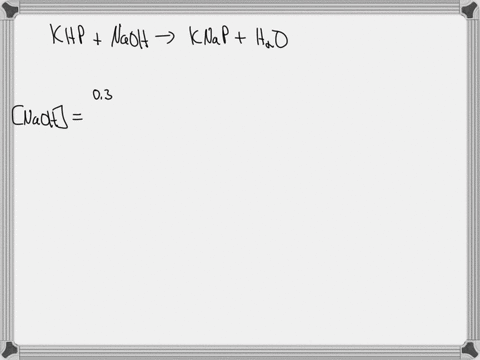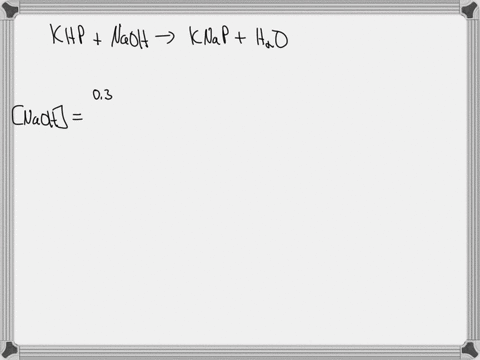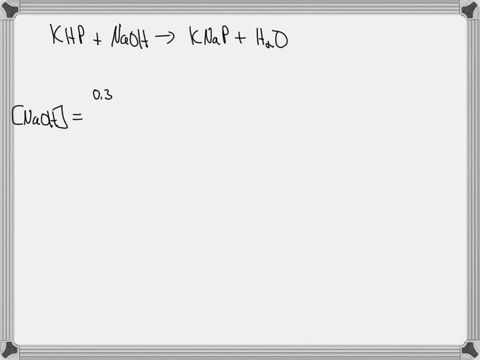Question
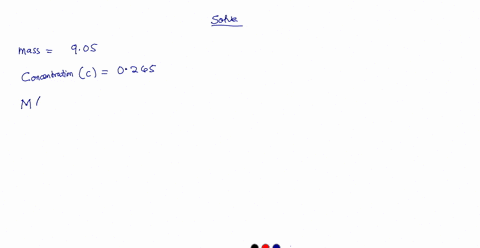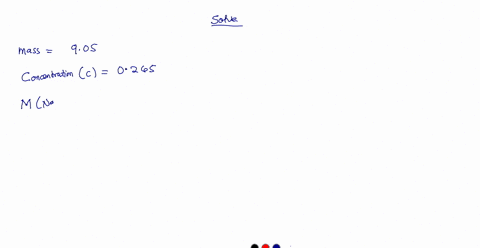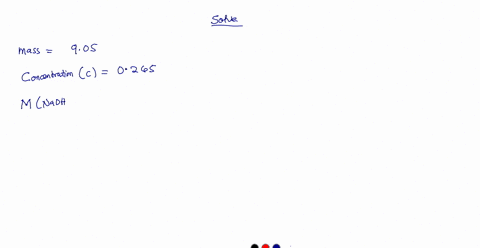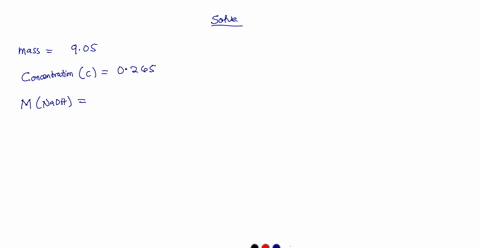
A 0.728 g sample of potassium acid phthalate requires 32.2 mL of an NaOH solution for neutralization. What is the molarity of the NaOH solution?
A 0.728 g sample of potassium acid phthalate requires 32.2 mL of an NaOH solution for neutralization. What is the molarity of the NaOH solution?
Added by Jes-S E.
Instant Answer
Step 1
First, we need to know the molar mass of potassium acid phthalate (KHP). The molar mass of KHP is approximately 204.22 g/mol. Show more…
Show all steps