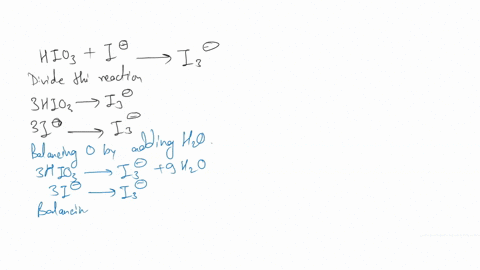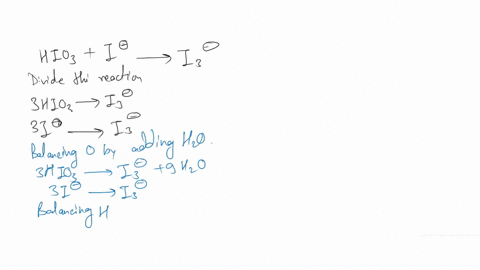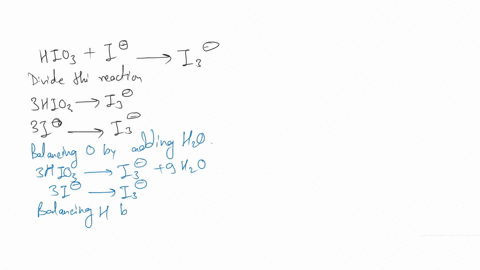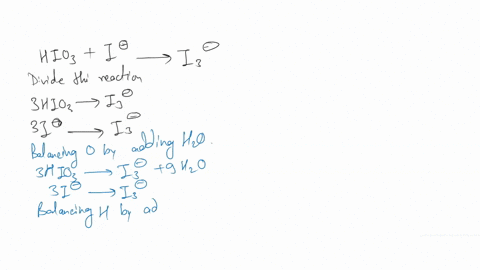Question
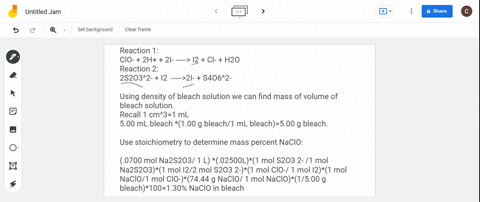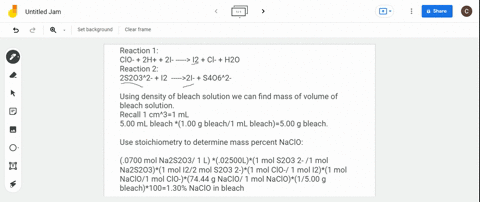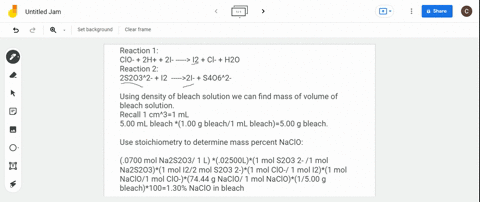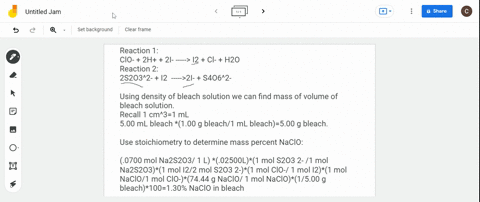
(a) Write a balanced redox equation for the reaction between iodine and thiosulfate ion, S2O3^2-. Thiosulfate ion, S2O3^2-, will be oxidized to the tetrathionate ion. (b) The concentration of commercial bleach can be estimated by the amount of free chlorine in the solution. 10.0 cm^3 of a sample of bleach was made up to a 250.0 cm^3 standard flask. 25.0 cm^3 of this solution were added to an excess of aqueous potassium iodide and reacted with 22.0 cm^3 of 0.105 mol dm^-3 thiosulfate solution. Calculate the concentration of chlorine in the diluted bleach solution.
(a) Write a balanced redox equation for the reaction between iodine and thiosulfate ion, S2O3^2-. Thiosulfate ion, S2O3^2-, will be oxidized to the tetrathionate ion.
(b) The concentration of commercial bleach can be estimated by the amount of free chlorine in the solution. 10.0 cm^3 of a sample of bleach was made up to a 250.0 cm^3 standard flask. 25.0 cm^3 of this solution were added to an excess of aqueous potassium iodide and reacted with 22.0 cm^3 of 0.105 mol dm^-3 thiosulfate solution. Calculate the concentration of chlorine in the diluted bleach solution.
Show more…

Added by F-Tima B.
Instant Answer
Step 1
### Part (a): Writing a Balanced Redox Equation ** Show more…
Show all steps