Question
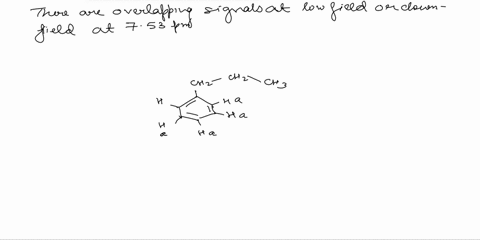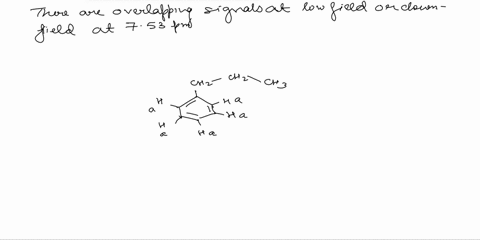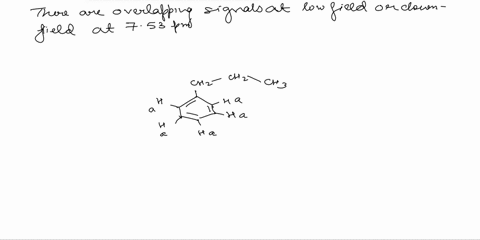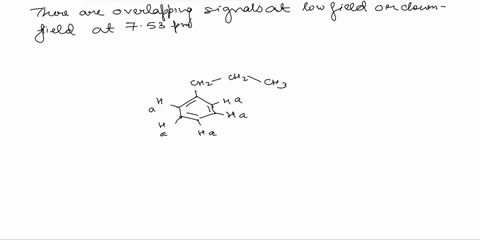
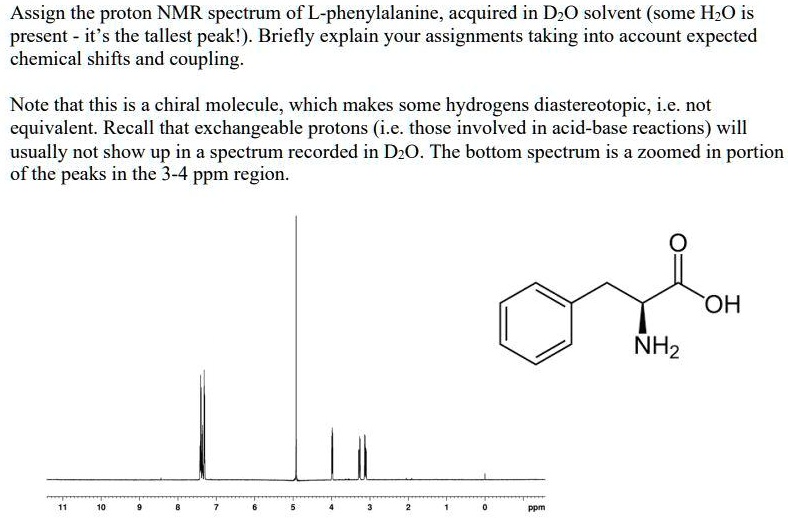
Assign the proton NMR spectrum of L-phenylalanine, acquired in D2O solvent (some H2O is present; it's the tallest peak!). Briefly explain your assignments, taking into account expected chemical shifts and coupling. Note that this is a chiral molecule, which makes some hydrogens diastereotopic, i.e., not equivalent. Recall that exchangeable protons (i.e., those involved in acid-base reactions) will usually not show up in a spectrum recorded in D2O. The bottom spectrum is a zoomed-in portion of the peaks in the 3-4 ppm region. OH NH2
Assign the proton NMR spectrum of L-phenylalanine, acquired in D2O solvent (some H2O is present; it's the tallest peak!). Briefly explain your assignments, taking into account expected chemical shifts and coupling.
Note that this is a chiral molecule, which makes some hydrogens diastereotopic, i.e., not equivalent. Recall that exchangeable protons (i.e., those involved in acid-base reactions) will usually not show up in a spectrum recorded in D2O. The bottom spectrum is a zoomed-in portion of the peaks in the 3-4 ppm region.
OH NH2
Show more…
Added by Brittany T.
Instant Answer
Step 1
First, we need to identify the different types of protons in L-phenylalanine. There are three main groups: the aromatic protons (5 protons), the alpha proton (1 proton), and the beta protons (2 protons). Show more…
Show all steps