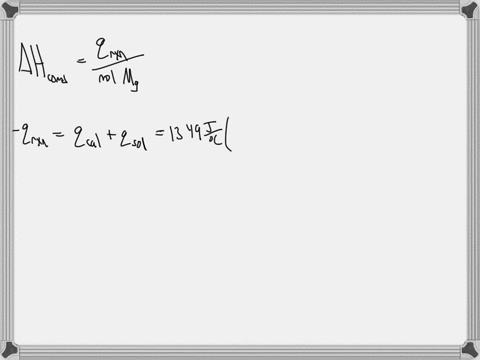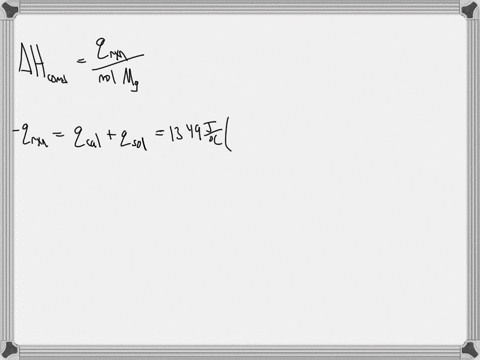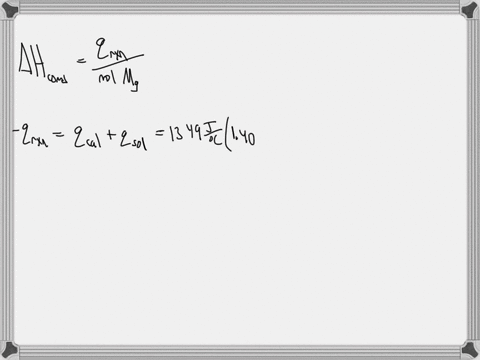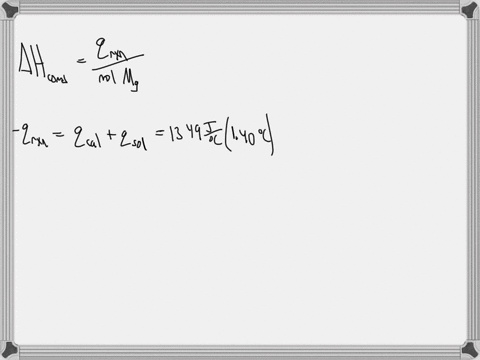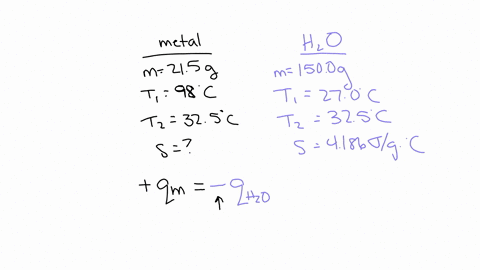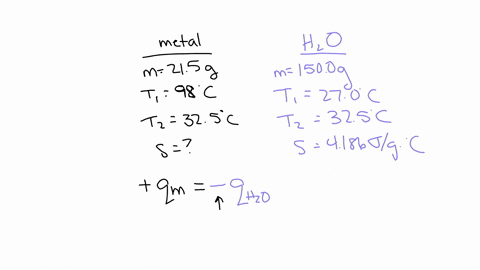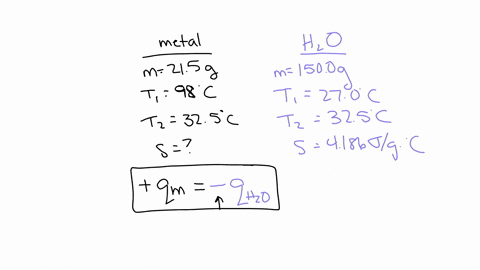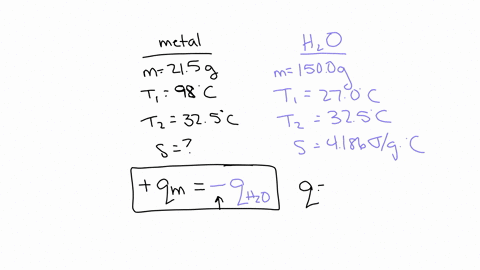Question
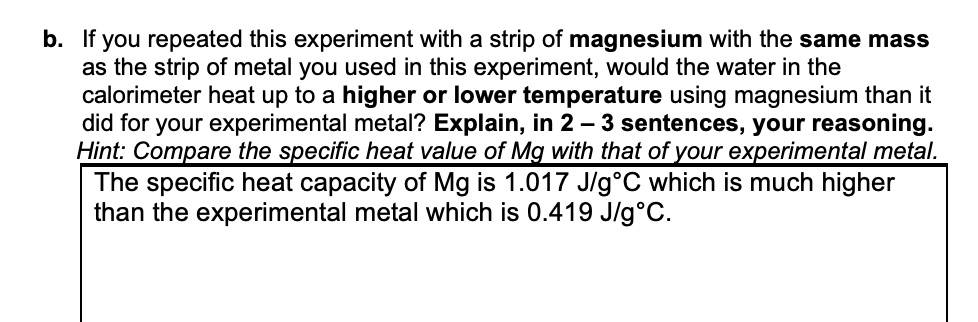
If you repeated this experiment with a strip of magnesium with the same mass as the strip of metal you used in this experiment, would the water in the calorimeter heat up to a higher or lower temperature using magnesium than it did for your experimental metal? Explain, in 2-3 sentences, your reasoning: Hint: Compare the specific heat value of Mg with that of your experimental metal. The specific heat capacity of Mg is 1.017 J/g°C which is much higher than the experimental metal which is 0.419 J/g°C.
If you repeated this experiment with a strip of magnesium with the same mass as the strip of metal you used in this experiment, would the water in the calorimeter heat up to a higher or lower temperature using magnesium than it did for your experimental metal? Explain, in 2-3 sentences, your reasoning: Hint: Compare the specific heat value of Mg with that of your experimental metal. The specific heat capacity of Mg is 1.017 J/g°C which is much higher than the experimental metal which is 0.419 J/g°C.
Show more…
Added by Christopher P.
Instant Answer
Step 1
If we used a strip of magnesium with the same mass as the experimental metal, the water in the calorimeter would heat up to a lower temperature. Show more…
Show all steps