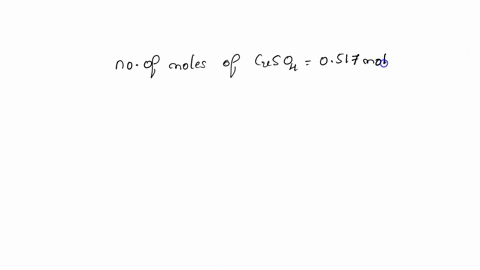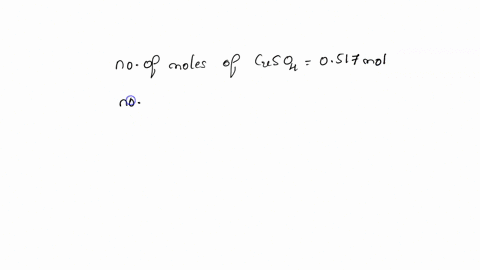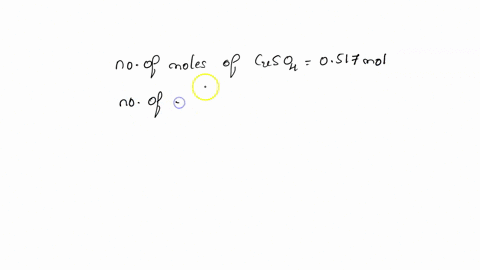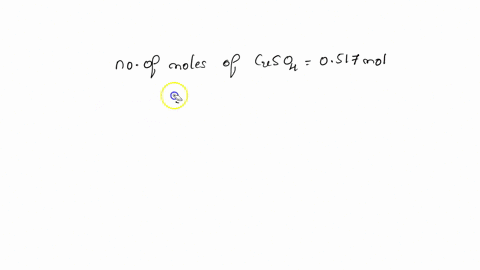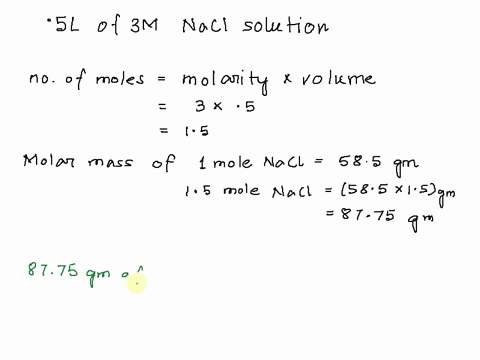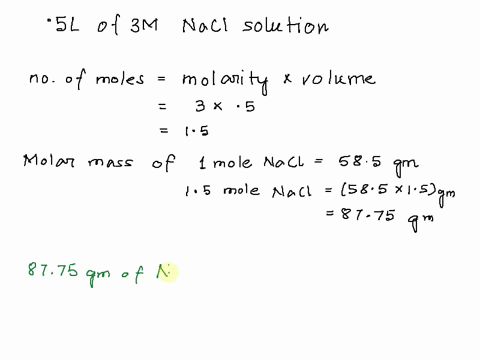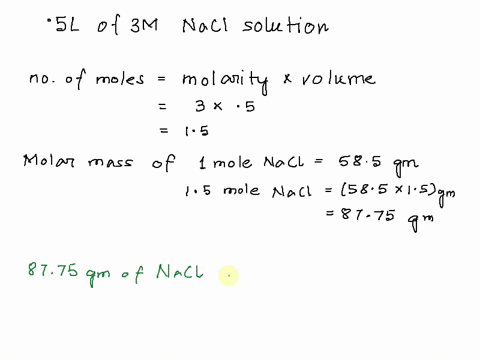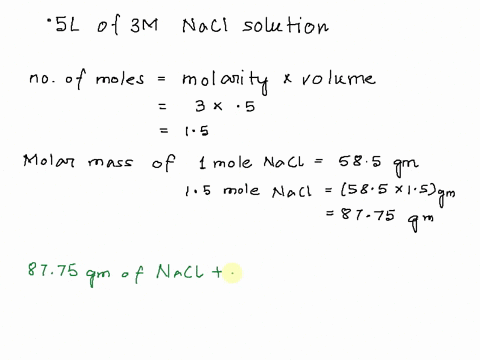Question
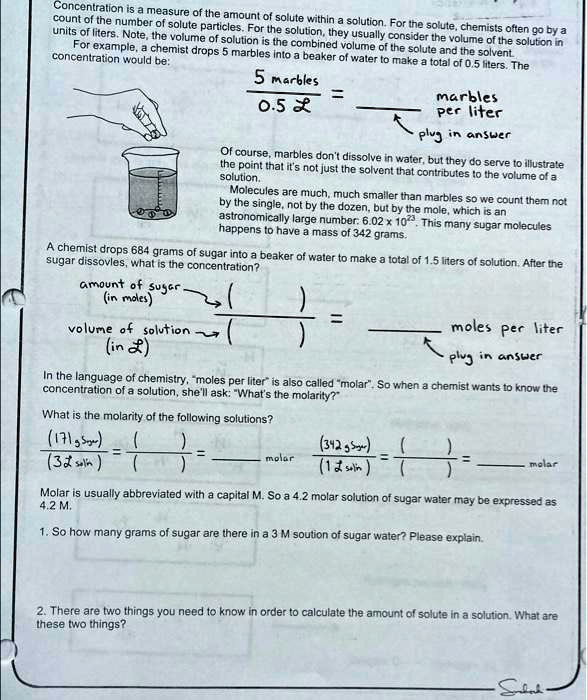
Concentration is the measure of the amount of solute within a unit of solvent. For a solution, chemists often go by liters. Note: the volume of solution usually considers the volume of the solute and the solvent combined. For example, if you combine 5 marbles with a beaker of water (solvent), the total volume would be 0.5 liters. The concentration of the marbles in the water would be 5 marbles / 0.5 liters = 10 marbles per liter. Of course, marbles don't dissolve in water, but they serve to illustrate that it's not just the solvent that contributes to the solution. The volume of solute molecules are much smaller than marbles, so we count them not by the dozen, but by the mole, which is an astronomically large number (6.02 * 10^23). This many molecules have a mass of 342 grams. If a chemist drops 684 grams of sugar into a beaker of water and it dissolves, what is the molarity of the solution? (moles of solute / volume of solution in liters) In the language of chemistry, "moles per liter" concentration is also called "molar". So when a chemist wants to know the concentration of a solution, she'll ask "What's the molarity?" Now, what is the molarity of the following solutions? (1.9 M, 3.5 M, 2.3 M) Molarity is usually abbreviated with a capital M. So a 42 M solution of sugar water may be expressed as 42M. So how many grams of sugar are there in a 42 M solution of sugar water? Please explain. There are two things you need to know in order to calculate the amount of solute in a solution. What are these two things?
Concentration is the measure of the amount of solute within a unit of solvent. For a solution, chemists often go by liters. Note: the volume of solution usually considers the volume of the solute and the solvent combined. For example, if you combine 5 marbles with a beaker of water (solvent), the total volume would be 0.5 liters. The concentration of the marbles in the water would be 5 marbles / 0.5 liters = 10 marbles per liter. Of course, marbles don't dissolve in water, but they serve to illustrate that it's not just the solvent that contributes to the solution. The volume of solute molecules are much smaller than marbles, so we count them not by the dozen, but by the mole, which is an astronomically large number (6.02 * 10^23). This many molecules have a mass of 342 grams. If a chemist drops 684 grams of sugar into a beaker of water and it dissolves, what is the molarity of the solution? (moles of solute / volume of solution in liters)
In the language of chemistry, "moles per liter" concentration is also called "molar". So when a chemist wants to know the concentration of a solution, she'll ask "What's the molarity?" Now, what is the molarity of the following solutions? (1.9 M, 3.5 M, 2.3 M)
Molarity is usually abbreviated with a capital M. So a 42 M solution of sugar water may be expressed as 42M.
So how many grams of sugar are there in a 42 M solution of sugar water? Please explain.
There are two things you need to know in order to calculate the amount of solute in a solution. What are these two things?
Show more…
Added by Frank C.
Instant Answer
Step 1
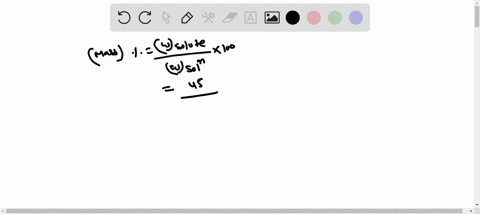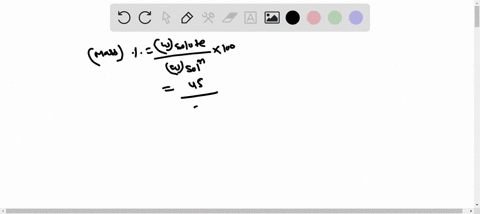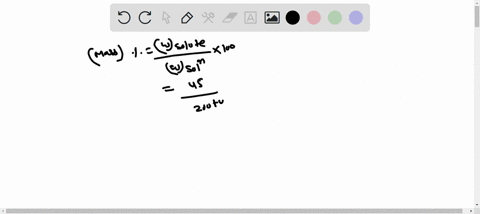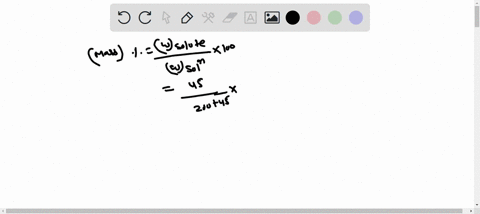
Molarity is defined as moles of solute per liter of solution. It tells you the concentration of the solute in the solution. Show more…
Show all steps