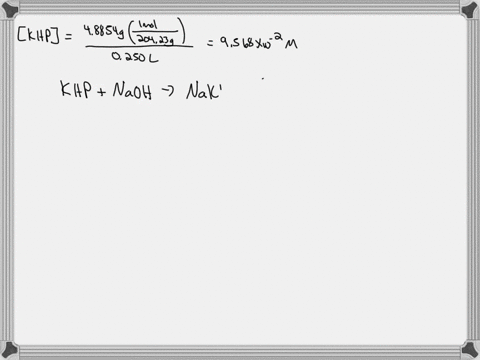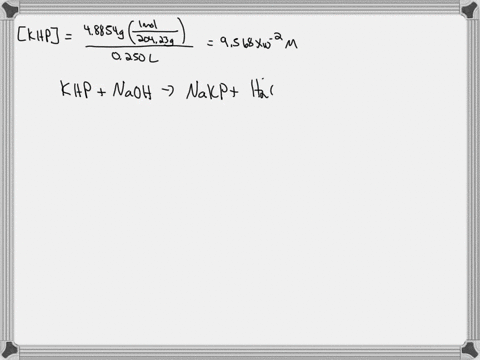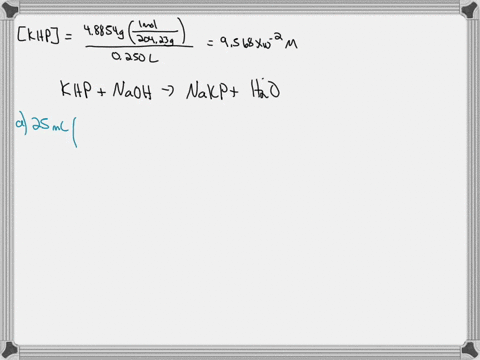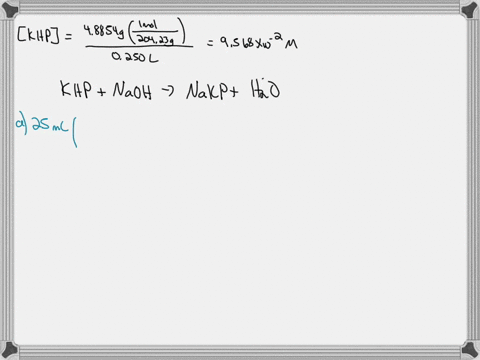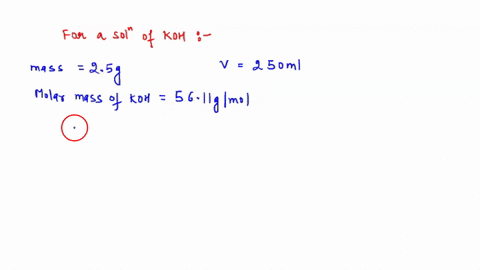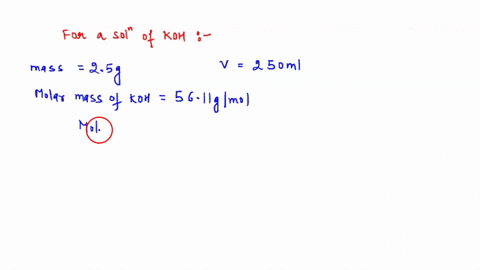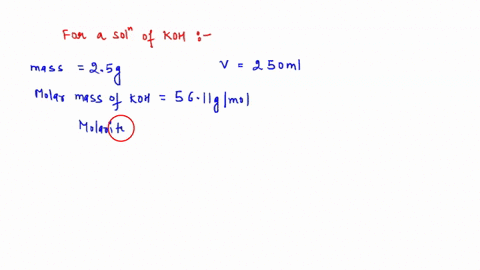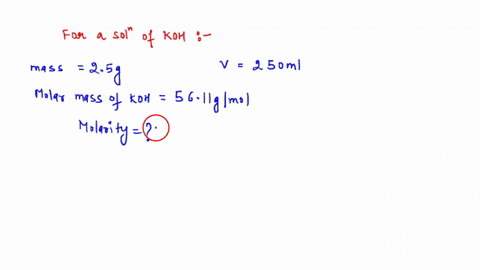Question
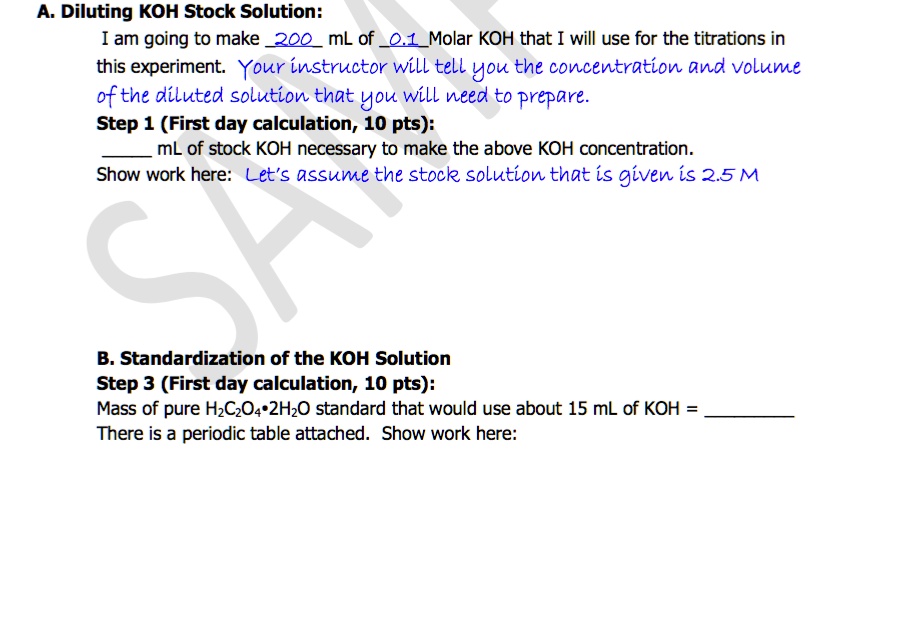
A. Diluting KOH Stock Solution: I am going to make _200_ mL of _0.1_ Molar KOH that I will use for the titrations in this experiment. Your instructor will tell you the concentration and volume of the diluted solution that you will need to prepare. Step 1 (First day calculation, 10 pts): ____ mL of stock KOH necessary to make the above KOH concentration. Show work here: Let's assume the stock solution that is given is 2.5 M B. Standardization of the KOH Solution Step 3 (First day calculation, 10 pts): Mass of pure $H_2C_2O_4\cdot2H_2O$ standard that would use about 15 mL of KOH = _____ There is a periodic table attached. Show work here:
A. Diluting KOH Stock Solution:
I am going to make _200_ mL of _0.1_ Molar KOH that I will use for the titrations in
this experiment. Your instructor will tell you the concentration and volume
of the diluted solution that you will need to prepare.
Step 1 (First day calculation, 10 pts):
____ mL of stock KOH necessary to make the above KOH concentration.
Show work here: Let's assume the stock solution that is given is 2.5 M
B. Standardization of the KOH Solution
Step 3 (First day calculation, 10 pts):
Mass of pure $H_2C_2O_4\cdot2H_2O$ standard that would use about 15 mL of KOH = _____
There is a periodic table attached. Show work here:
Show more…
Added by Josefa W.
Instant Answer
Step 1
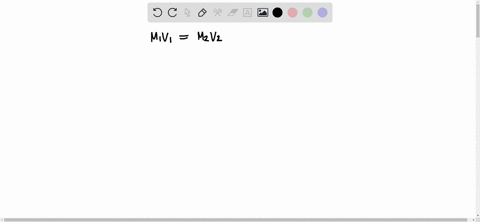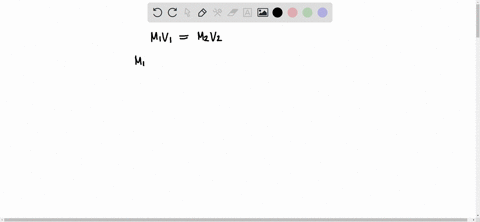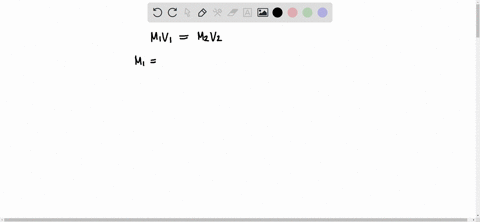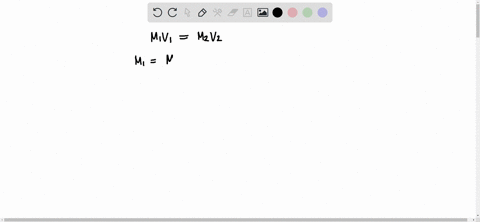
The formula to use here is M1V1 = M2V2, where M1 is the molarity of the stock solution, V1 is the volume of the stock solution we need to find, M2 is the molarity of the diluted solution, and V2 is the volume of the diluted solution. Assuming that the desired Show more…
Show all steps