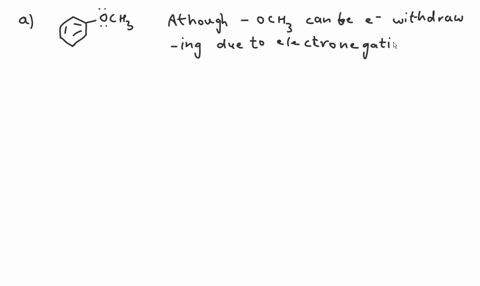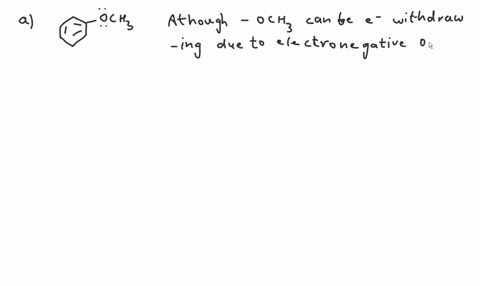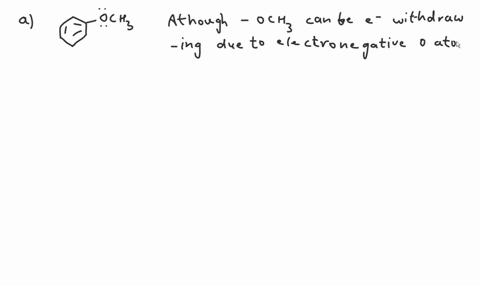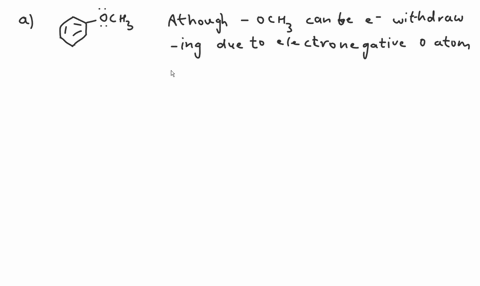Question
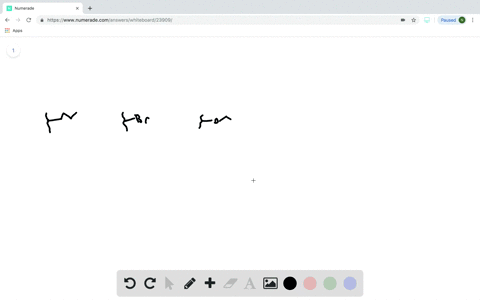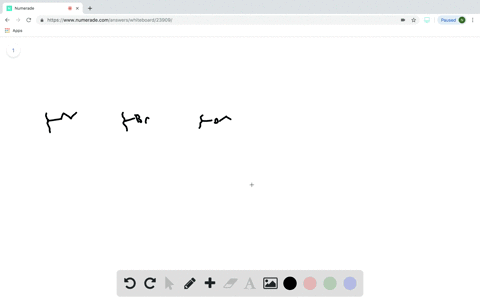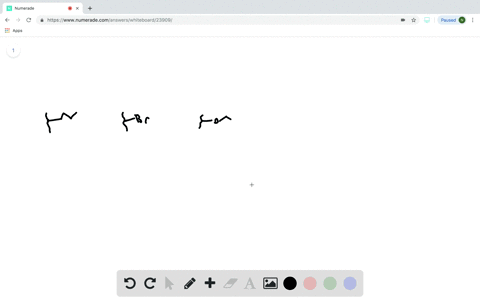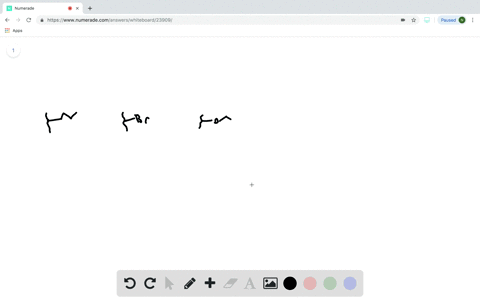
Does the substituent F- have an electron-withdrawing or an electron-donating inductive effect? Select the single best answer. Electron-donating effect Electron-withdrawing effect
Does the substituent F- have an electron-withdrawing or an electron-donating inductive effect? Select the single best answer.
Electron-donating effect
Electron-withdrawing effect

Added by Phillip Q.
Instant Answer
Step 1
This extra electron can be thought of as an electron cloud that can interact with nearby atoms or groups. In the case of F-, the fluorine atom is more electronegative than carbon, so it will pull electron density away from the carbon atom it is attached to. This Show more…
Show all steps