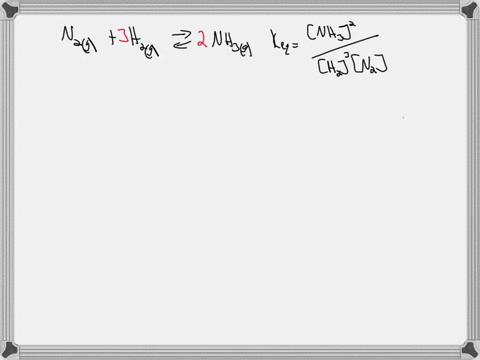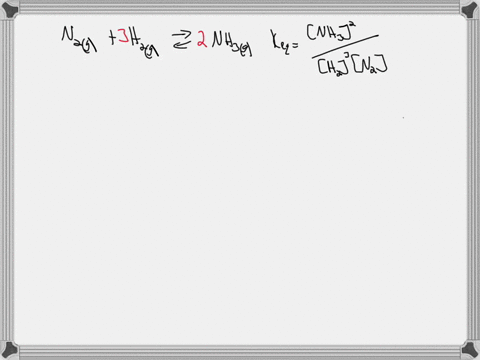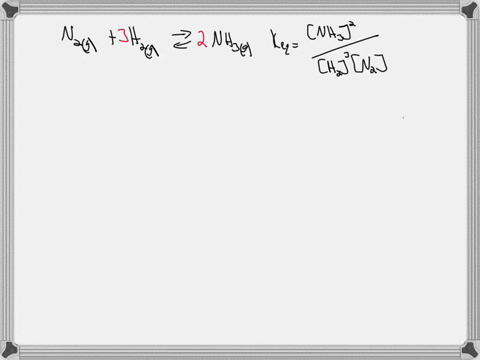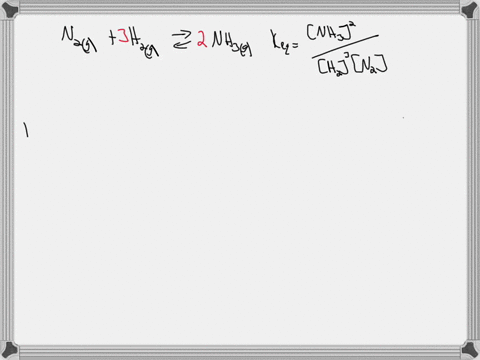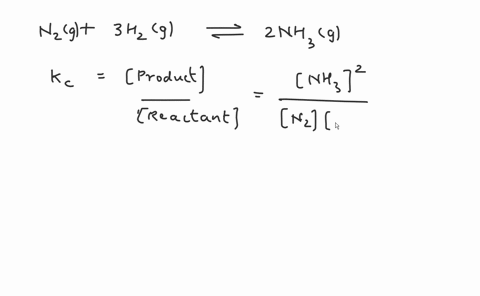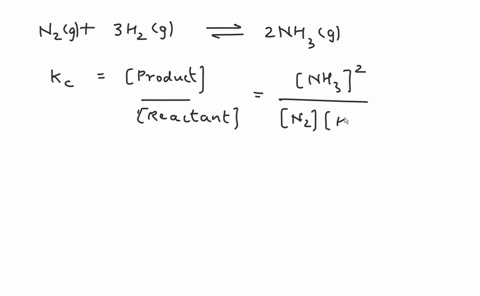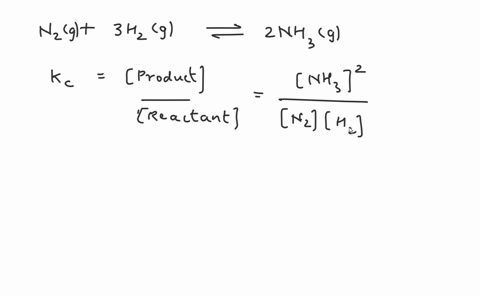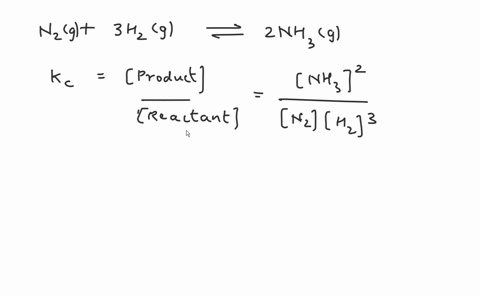Question
For the Haber reaction of nitrogen gas reacting with hydrogen gas to yield ammonia gas at 298.15 K, what is the log10 of the equilibrium constant for this reaction? If the partial pressures of these gases at equilibrium are as follows: N2 = 0.02 atm, H2 = 0.01 atm, NH3 = 0.11 atm.
For the Haber reaction of nitrogen gas reacting with hydrogen gas to yield ammonia gas at 298.15 K, what is the log10 of the equilibrium constant for this reaction? If the partial pressures of these gases at equilibrium are as follows: N2 = 0.02 atm, H2 = 0.01 atm, NH3 = 0.11 atm.
Show more…

Added by Scott W.
Instant Answer
Step 1
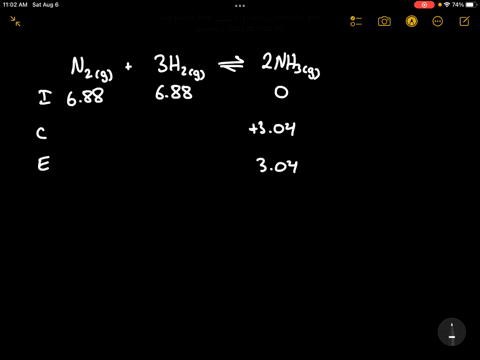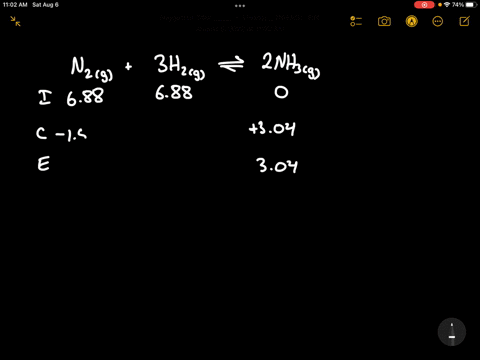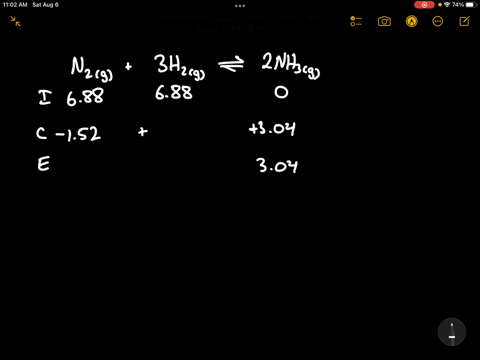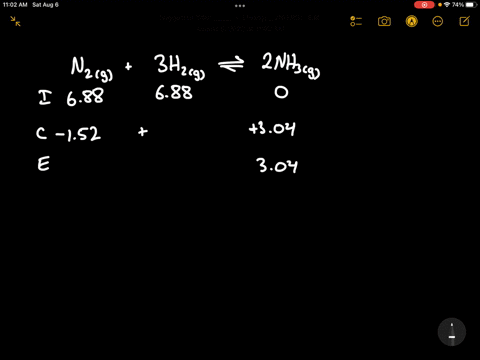
Write the balanced chemical equation for the Haber process: N2(g) + 3H2(g) ⇌ 2NH3(g) Show more…
Show all steps