0:00
Hello students.
00:01
So in this question you have been asked about in which of the following will neptylene dissolve? see we have here three options.
00:08
First one is n -a -o -h and the second is h -c -l and the third one is diethyl ether.
00:18
Diethyl ether.
00:21
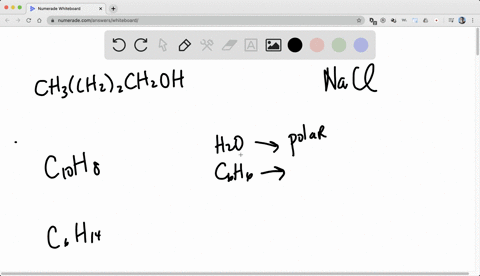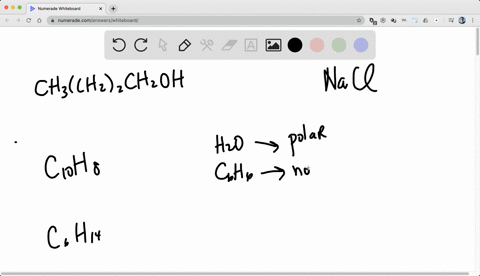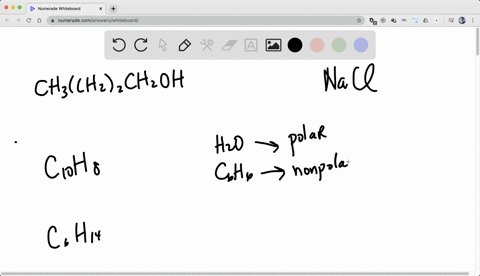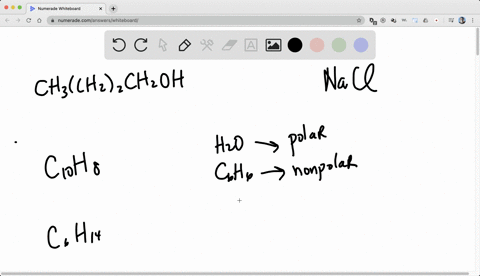
So for solubility, what pact do we follow? we follow the pack of like dissolves like.
00:35
Alright, so like dissolves like means if we have the two categories of generally.
00:42
One is non -polar, known polar and the another one is polar.
00:49
So if we want to dissolve a known polar substance or the known -polar chemical, we need non -polar solvent.
00:56
And if we want to dissolve a polar substance or the polar chemical, we need a polar solvent.
01:01
Right, so like dissolves, so known -polar will dissolve non -polar and polar will dissolve polar.
01:06
So if you talk about neptylene, if you talk about neptylene over here, neptylene is what? it is an organic compound, organic compound, right? so for organic compound, we know that they are basically, they are generally non -polar in nature...