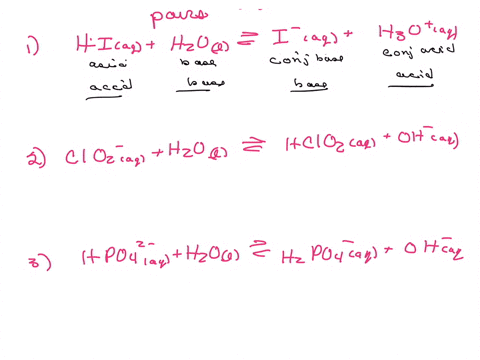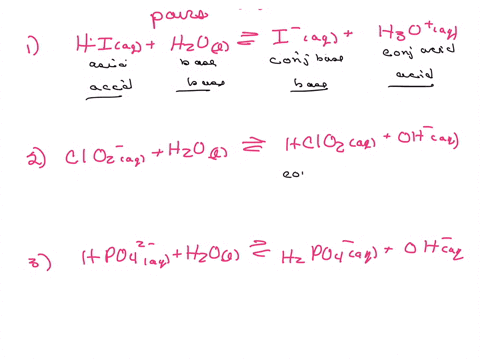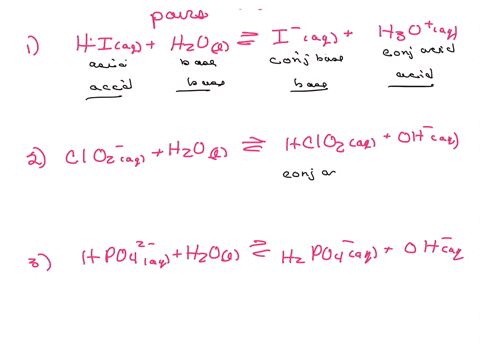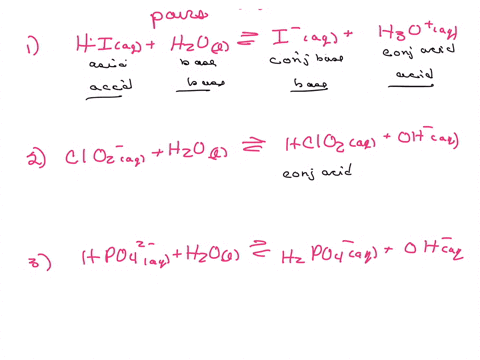Question
Label the acid and base and the conjugate acid and base in the following reactions. Use curved arrow notations to show the movement of electron pairs. (20) CN- + H2O -> HCN + OH- H2SO4 + Cl- -> HCl + HSO4- O2 + H2O -> H2O2 HS- + OH- -> H2S + SO4^2- NH3 + H2O -> NH4+ + OH- Calculate the equilibrium constant for the reaction of a sparingly soluble calcium phosphate when the given equilibrium constant is 2x10^-29. (20) Write the conjugate acid for the following bases. (20 points) (a) NH3 (b) HO- (c) CH3O- (d) Cl- Write the conjugate base for the following acids. (20 points) (a) HBr (b) HSO4- (c) CHO- (d) NH4+ Write down the expression for the equilibrium constant for the following reaction. (20) 2NO2(g) + 2O2(g) -> 2N2O5(g) Which of the following reactions will shift left when the temperature is decreased? (20) (a) 2NO2(g) + O2(g) -> 2N2O5(g) + heat (b) 2NH3(g) -> N2(g) + 3H2(g) + heat (c) CO(g) + H2(g) -> CH3OH(g) + heat (d) N2(g) + 3H2(g) -> 2NH3(g) + heat (e) 2H2S(g) + 3O2(g) -> 2SO2(g) + 2H2O(g) + heat (f) CH4(g) + 2AgNO3(aq) -> 2Ag(s) + CO2(g) + 2H2O(g) Distinguish between the following: (20) (a) Acid and base (b) Conjugate acid and conjugate base
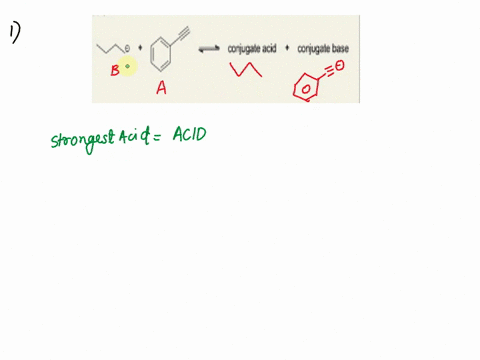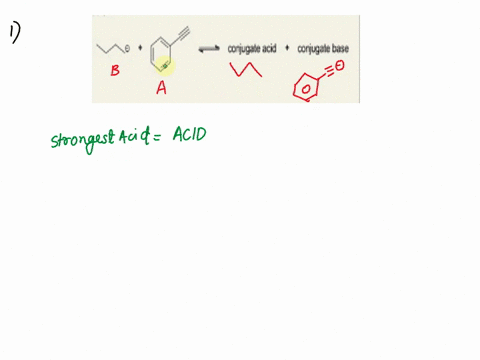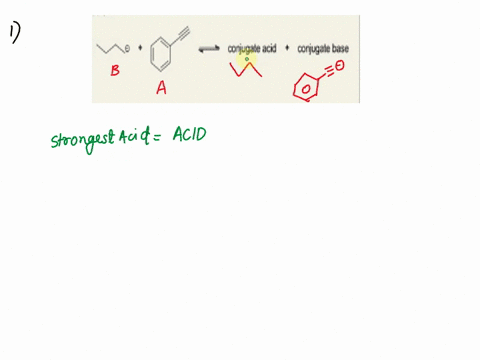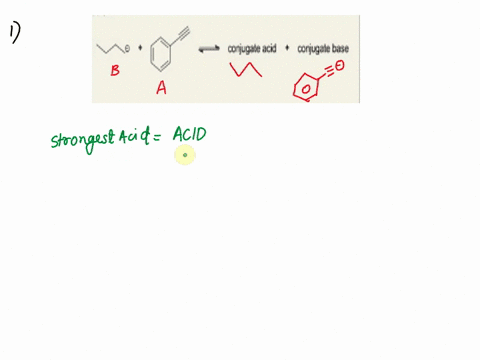
Label the acid and base and the conjugate acid and base in the following reactions. Use curved arrow notations to show the movement of electron pairs. (20)
CN- + H2O -> HCN + OH-
H2SO4 + Cl- -> HCl + HSO4-
O2 + H2O -> H2O2
HS- + OH- -> H2S + SO4^2-
NH3 + H2O -> NH4+ + OH-
Calculate the equilibrium constant for the reaction of a sparingly soluble calcium phosphate when the given equilibrium constant is 2x10^-29. (20)
Write the conjugate acid for the following bases. (20 points)
(a) NH3
(b) HO-
(c) CH3O-
(d) Cl-
Write the conjugate base for the following acids. (20 points)
(a) HBr
(b) HSO4-
(c) CHO-
(d) NH4+
Write down the expression for the equilibrium constant for the following reaction. (20)
2NO2(g) + 2O2(g) -> 2N2O5(g)
Which of the following reactions will shift left when the temperature is decreased? (20)
(a) 2NO2(g) + O2(g) -> 2N2O5(g) + heat
(b) 2NH3(g) -> N2(g) + 3H2(g) + heat
(c) CO(g) + H2(g) -> CH3OH(g) + heat
(d) N2(g) + 3H2(g) -> 2NH3(g) + heat
(e) 2H2S(g) + 3O2(g) -> 2SO2(g) + 2H2O(g) + heat
(f) CH4(g) + 2AgNO3(aq) -> 2Ag(s) + CO2(g) + 2H2O(g)
Distinguish between the following: (20)
(a) Acid and base
(b) Conjugate acid and conjugate base
Show more…

Added by Gary B.
Instant Answer
Step 1
Label the acid and base and the conjugate acid and base in the following reactions: a) HCN + H2O -> CN- + H3O+ Acid: HCN, Base: H2O, Conjugate Acid: H3O+, Conjugate Base: CN- b) HCl + OH- -> Cl- + H2O Acid: HCl, Base: OH-, Conjugate Acid: H2O, Conjugate Base: Show more…
Show all steps