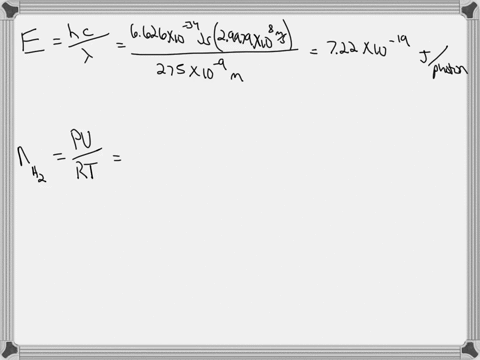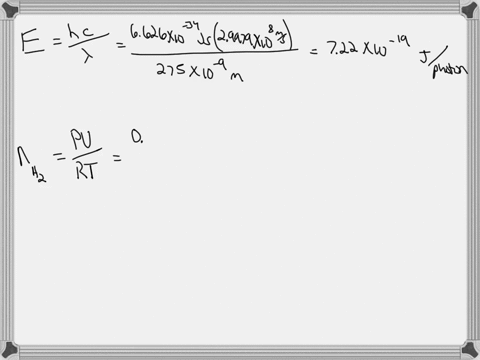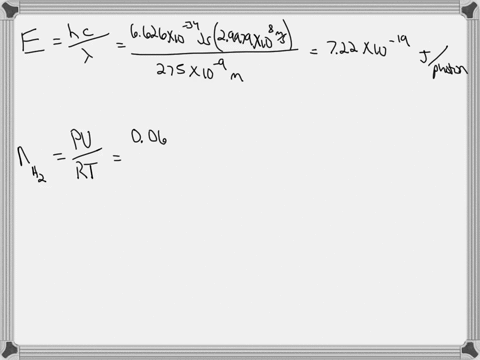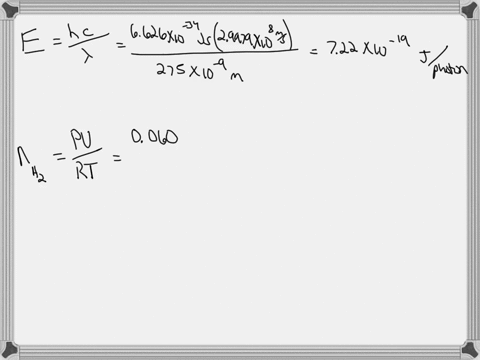Question
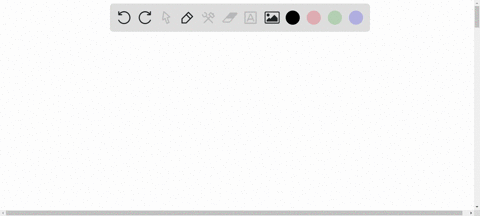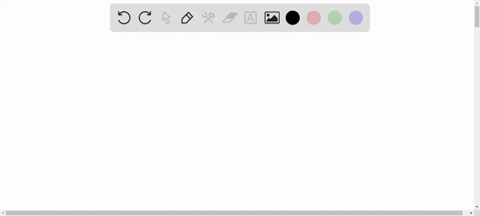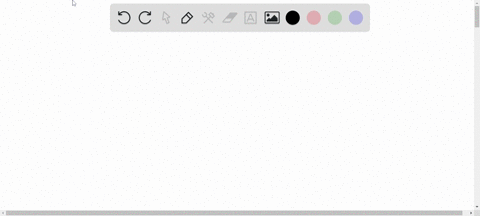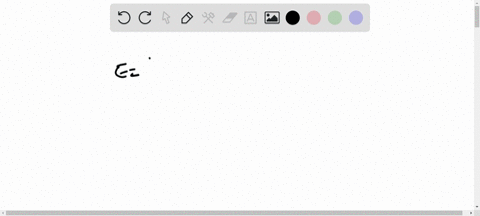
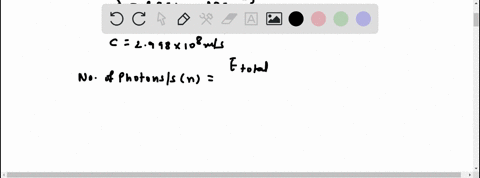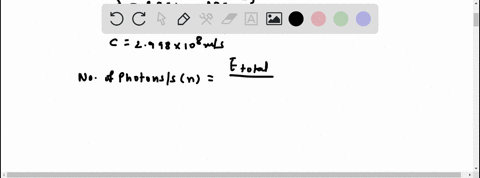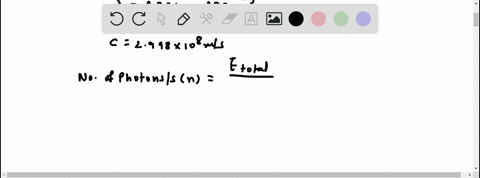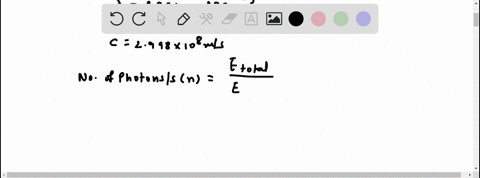
Light of wavelengths shorter than 275 nm can be used to photodissociate the hydrogen molecule into hydrogen atoms in the gas phase S0. mL glass cylinder contains Hz (g) at 45.0 mtorr id 25 %C. What Minimum amount of light energy must be absorbed by Ihe hydrogen in Ihe (ube t0 dissociate 44.0G of the molecules? energy absorbed:
Light of wavelengths shorter than 275 nm can be used to photodissociate the hydrogen molecule into hydrogen atoms in the gas phase S0. mL glass cylinder contains Hz (g) at 45.0 mtorr id 25 %C. What Minimum amount of light energy must be absorbed by Ihe hydrogen in Ihe (ube t0 dissociate 44.0G of the molecules?
energy absorbed:
Show more…

Added by Julian A.
Instant Answer
Step 1
First, we need to calculate the number of moles in 44.0 g of hydrogen gas (H2). The molar mass of H2 is approximately 2 g/mol, so 44.0 g of H2 is 22.0 moles. Show more…
Show all steps