Question
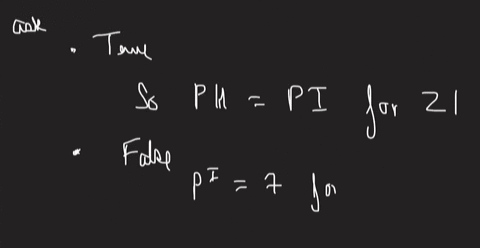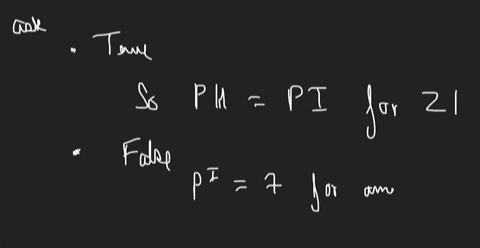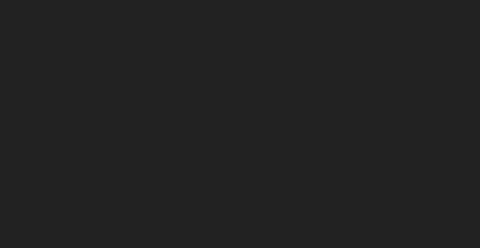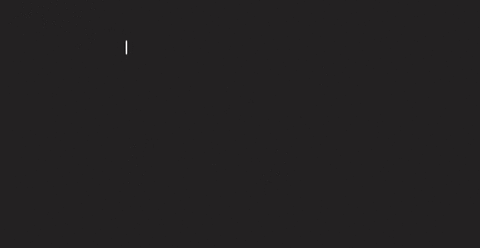
An amino acid is dissolved in solution at a pH which is the same as its isoelectric point. Most of the amino acid molecules in solution will have an ionized carboxylic acid group. True or False? Most of the amino acid molecules in solution will have an ionized amine group. True or False? Most of the amino acid molecules in solution will exhibit a net charge of zero. True or False? Lowering the pH will lower the zwitterion concentration. True or False? Liquid X has a surface tension value of 35 mNm and liquid Y has a surface tension value of 50 mNm. The interfacial tension between the two liquids could be determined using the capillary rise method. True or False? The cohesive forces in liquid Y are stronger than the cohesive forces in liquid X. True or False? X will spread on Y more readily than it will spread on X. True or False? The work of adhesion is the work required to separate liquid X from liquid Y at their interface. True or False?
An amino acid is dissolved in solution at a pH which is the same as its isoelectric point. Most of the amino acid molecules in solution will have an ionized carboxylic acid group. True or False? Most of the amino acid molecules in solution will have an ionized amine group. True or False? Most of the amino acid molecules in solution will exhibit a net charge of zero. True or False? Lowering the pH will lower the zwitterion concentration. True or False?
Liquid X has a surface tension value of 35 mNm and liquid Y has a surface tension value of 50 mNm. The interfacial tension between the two liquids could be determined using the capillary rise method. True or False? The cohesive forces in liquid Y are stronger than the cohesive forces in liquid X. True or False? X will spread on Y more readily than it will spread on X. True or False? The work of adhesion is the work required to separate liquid X from liquid Y at their interface. True or False?
Show more…

Added by Irene C.
Instant Answer
Step 1
At this pH, the carboxylic acid group will be deprotonated and the amine group will be protonated, so most of the amino acid molecules in solution will have an ionized carboxylic acid group and a protonated amine group. Therefore, the statement "Most of the amino Show more…
Show all steps