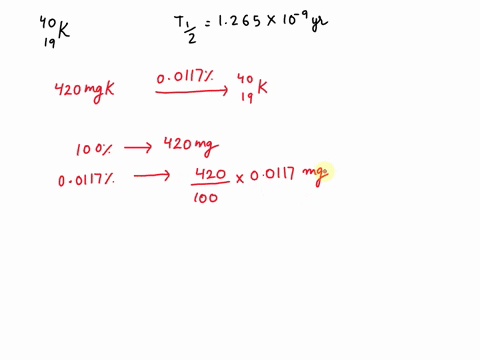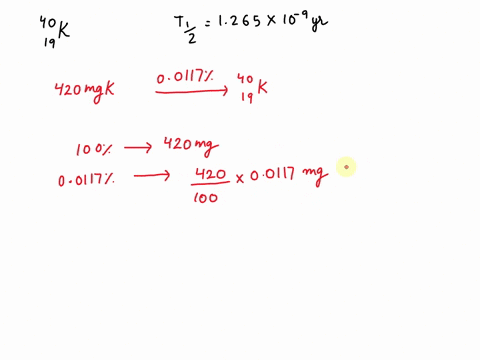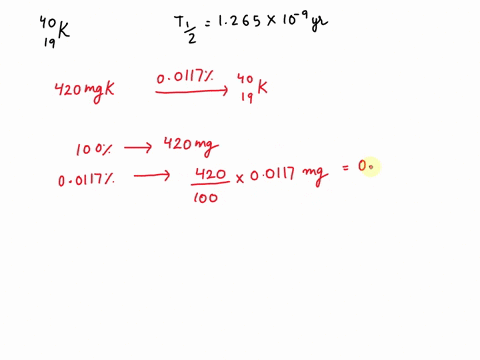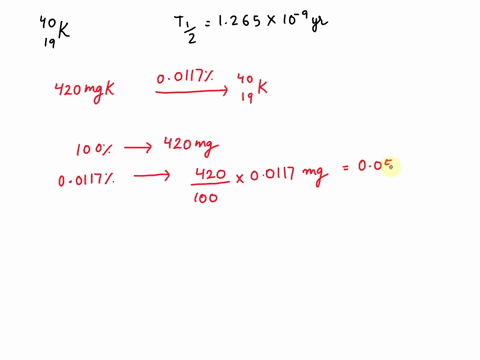Question
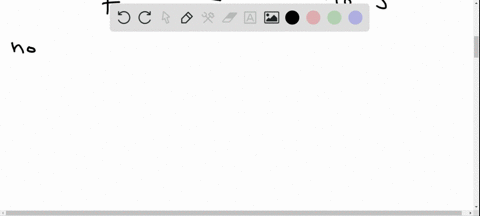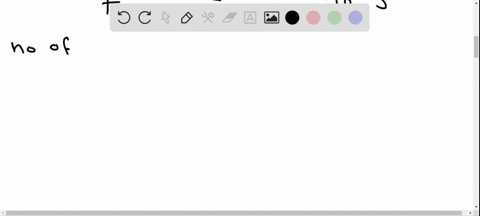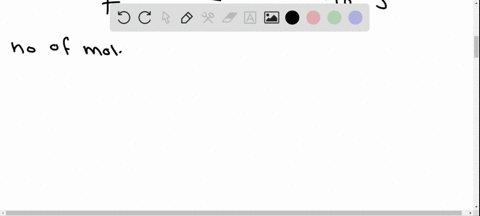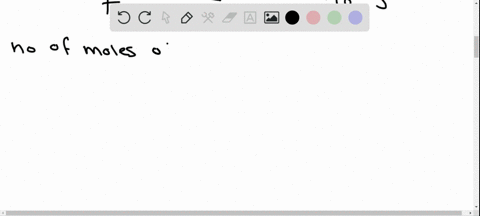
Potassium has a radioactive isotope, K-40, with a half-life of 1.25 billion years, and a natural abundance of 0.012% (120 parts per million). Sea-water contains about 380 ppm of potassium (0.38 grams per litre), which is essential for life. Calculate the radioactivity of sea-water due to the potassium, giving your answer in Bq m−3 .
Potassium has a radioactive isotope, K-40, with a half-life of 1.25 billion years, and a natural abundance of 0.012% (120 parts per million). Sea-water contains about 380 ppm of potassium (0.38 grams per litre), which is essential for life. Calculate the radioactivity of sea-water due to the potassium, giving your answer in Bq m−3
.
Show more…
Added by Bob J.
Instant Answer
Step 1
Given that sea-water contains about 380 ppm of potassium, this means there are 380 milligrams of potassium per liter of sea-water. Show more…
Show all steps