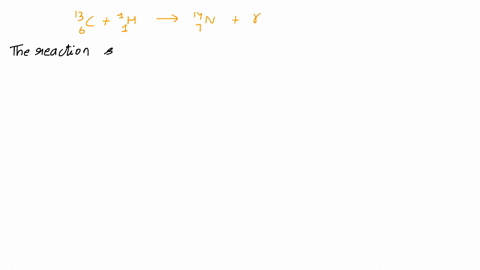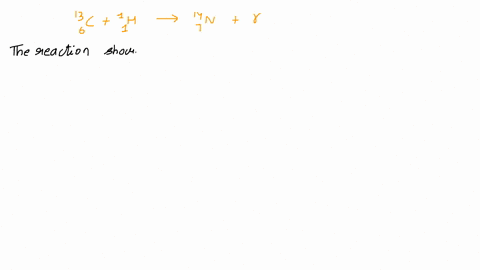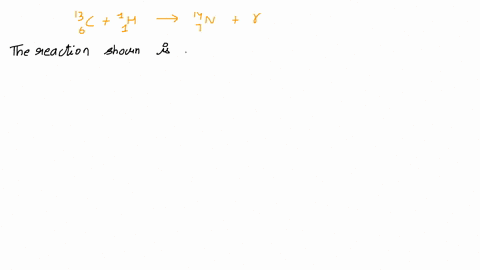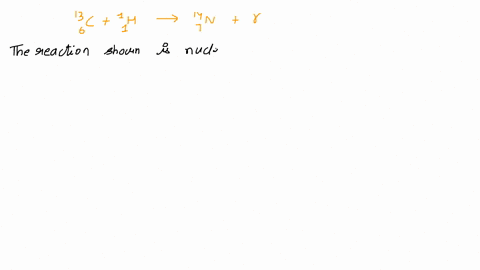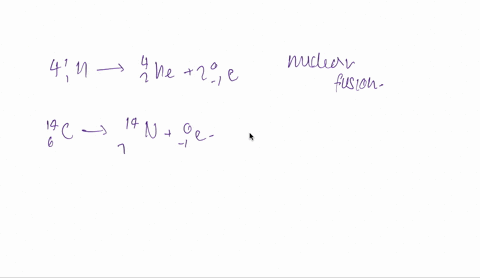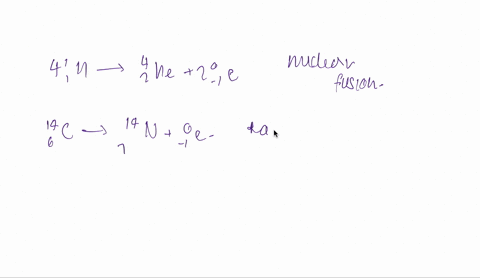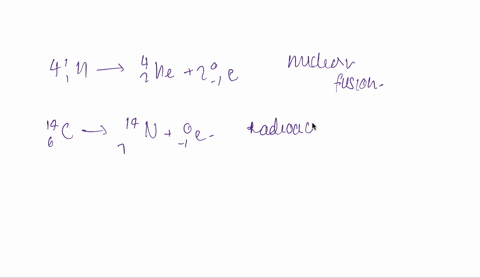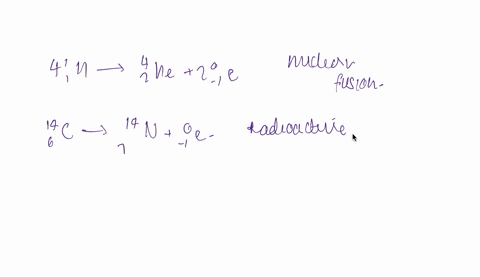00:01
Hello students, welcome here in this question we have given some radio active reactions are given.
00:06
So alpha, beta and gamma emissions are takes place.
00:10
So we have to complete the reactions and give the type of reaction taking place.
00:16
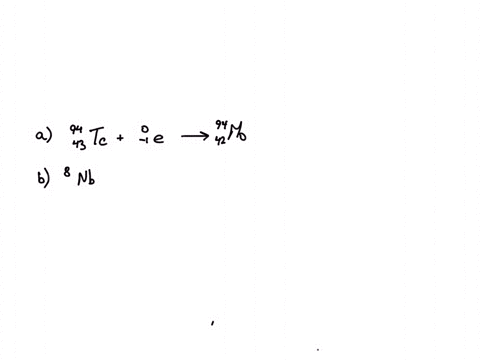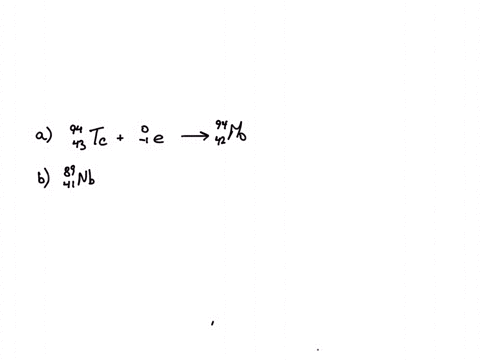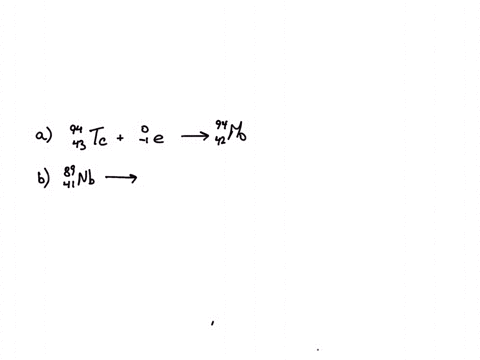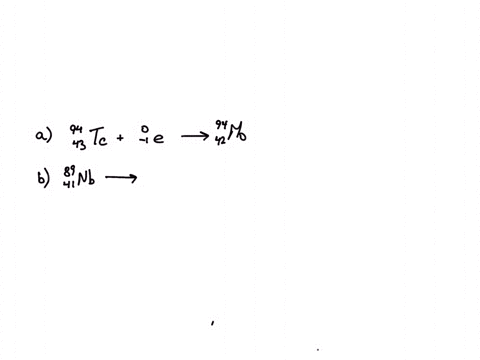
So the first one is potassium 1942, this tries to emission of one electron.
00:26
So this one is plus calcium.
00:30
Calcium 20 and 42 so it is a type of reaction which is beta emission so here it is beta emission taking place and the second one is giving here plutonium 94 239 gives rise to helium is given out means it is alpha emission so uranium 92 to 235 is a product so it is alpha emission okay and the third one is here uranium 92 235 gives rise to helium 24 then we will get indium sorry thorium 30m okay, so it is also alpha emission.
01:40
And the fourth one is hydrogen 1 -1 plus hydrogen -13.
01:47
So both are isotopes are combinedly give 2 for helium.
01:54
So it is fusion.
01:58
Fusion is taking place here.
02:01
And the third one, fifth one is here.
02:05
Chithium 3, 6 plus neutron bombardment is taking place.
02:12
So helium is out and plus hydrogen 1 3.
02:17
Chitrium is formed.
02:19
So this one is fission.
02:22
And here the sixth one is aluminum 1327 giving bombardment with 2 4 alpha particles.
02:35
Then fast press 15 and 30 is giving and 1 neutron is emitted so here it is artificial transformation artificial transformation is taking place and the 7th one is given that here 7th 1 beryllium 4 9 plus hydrogen hydrogen 1 -1 gives rise to lithium 3 6 plus helium -24, which is alpha emission.
03:24
And the 8th 1 is potassium 19 gives rise to 1 positron out and argon 18 and 37 is forming.
03:38
So it is positron emission.
03:47
Emission is taking place here.
03:51
And the ninth one is uranium 92 -2 -235 neutron bombardment is taking place.
03:59
So here we will get barium 56 -142 plus krypton -3691 plus 3 neutrons.
04:12
Okay, so it is fission.
04:16
So fission of the uranium molecule item is taking place here.
04:22
And the 10th one is uranium 92 to 338...