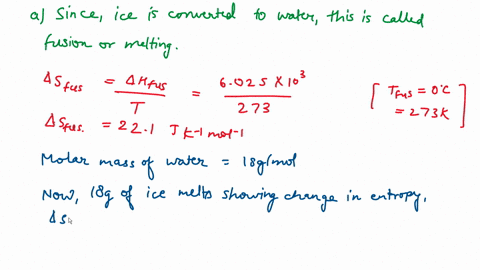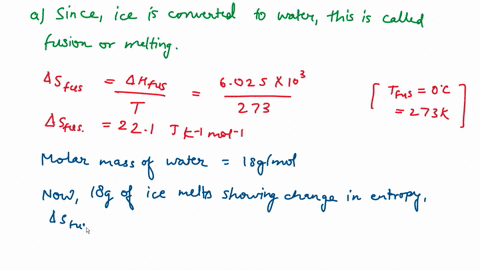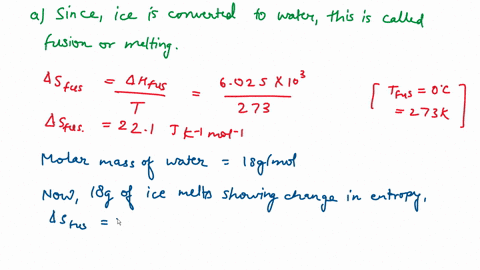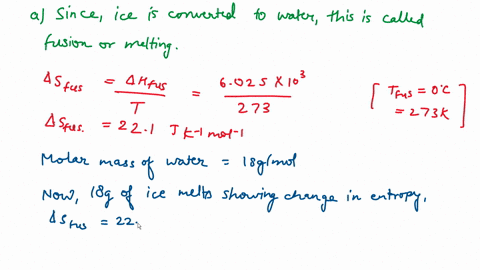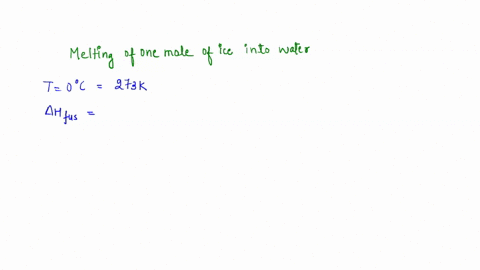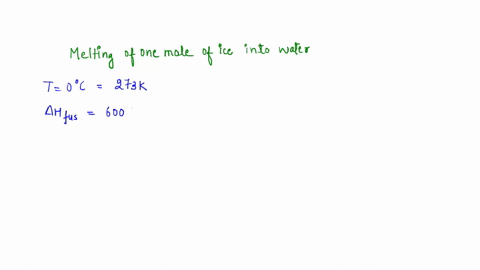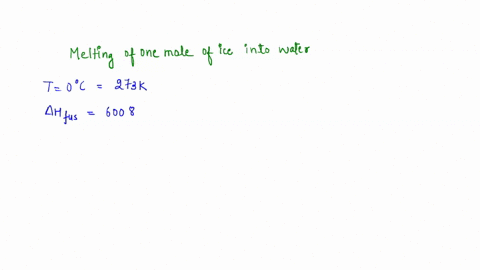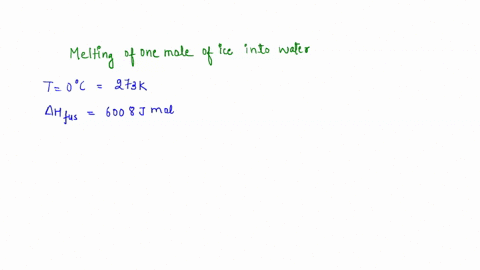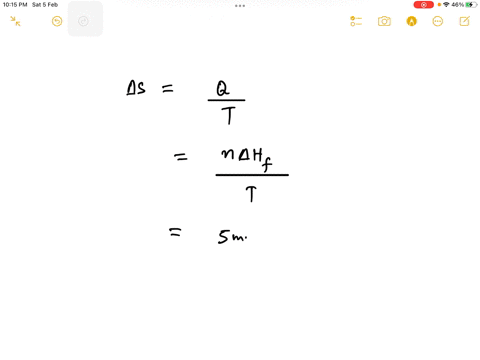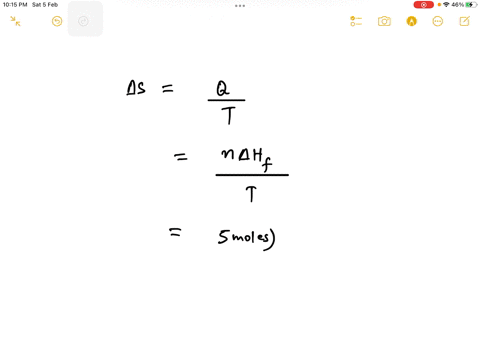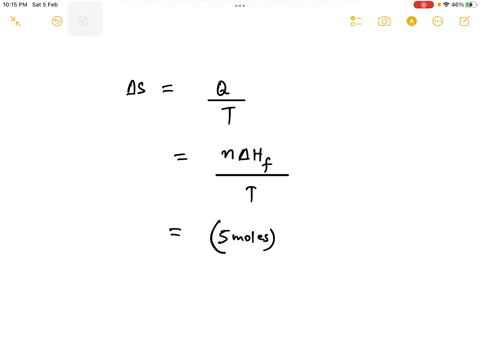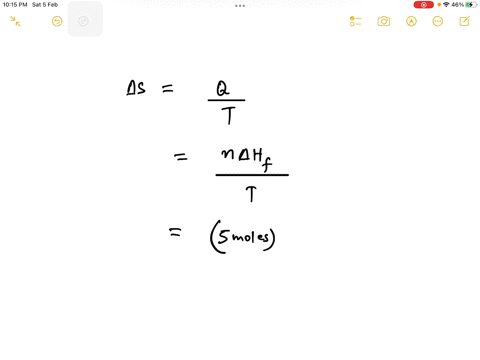Question
Question A25 Calculate the molar entropy change for the melting of ice, H?O(s), to liquid water, H?O(l) at a temperature of 0 °C and pressure of 1.00 atm. H?O (s) \(\rightleftharpoons\) H?O (l) The molar enthalpy change for this process is 6.0 kJ mol?¹ A. 3.8 J K?¹ mol?¹ B. -22 J K?¹ mol?¹ C. 22 J K?¹ mol?¹ D. J K?¹ mol?¹ E. 0.32 J K?¹ mol?¹ (3 marks)
Question A25
Calculate the molar entropy change for the melting of ice, H?O(s), to liquid water, H?O(l) at a
temperature of 0 °C and pressure of 1.00 atm.
H?O (s) \(\rightleftharpoons\) H?O (l)
The molar enthalpy change for this process is 6.0 kJ mol?¹
A.
3.8 J K?¹ mol?¹
B.
-22 J K?¹ mol?¹
C. 22 J K?¹ mol?¹
D.
J K?¹ mol?¹
E.
0.32 J K?¹ mol?¹
(3 marks)
Show more…

Added by Patricia T.
Instant Answer
Step 1
Since 1 kJ = 1000 J, the enthalpy change is 6.0 kJ/mol * 1000 J/kJ = 6000 J/mol. Show more…
Show all steps