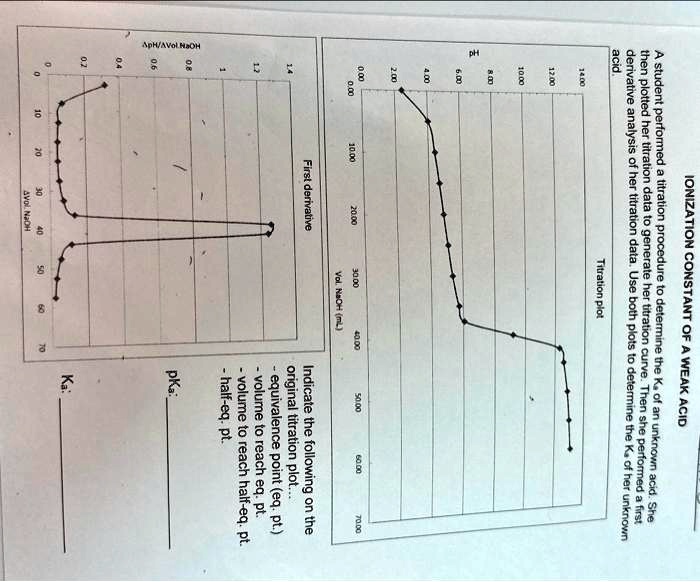
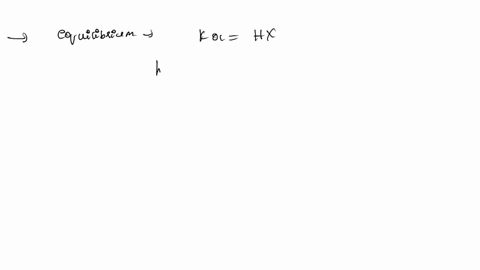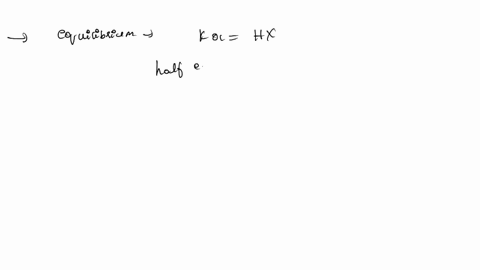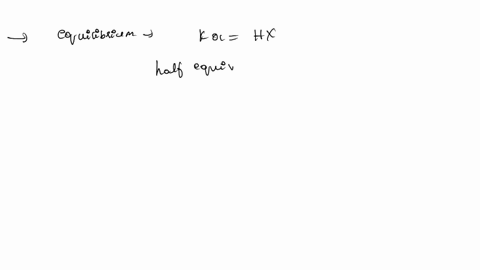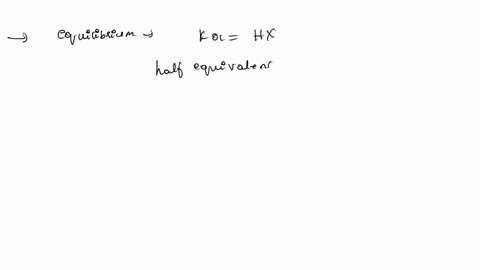Question
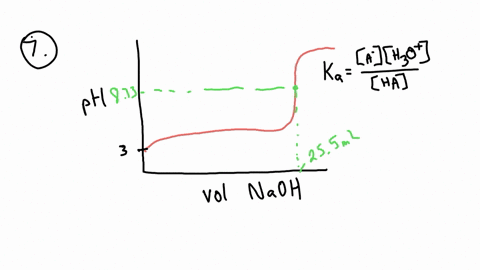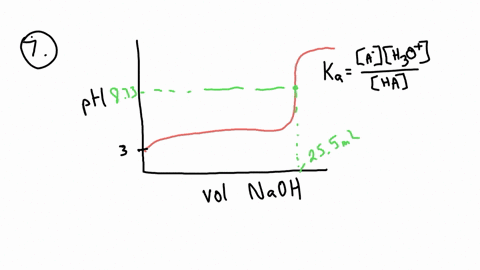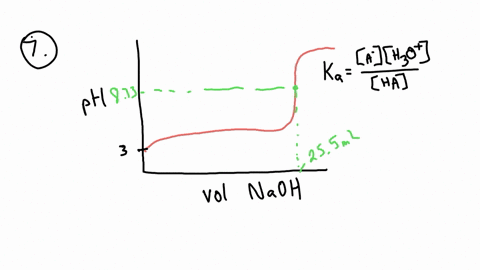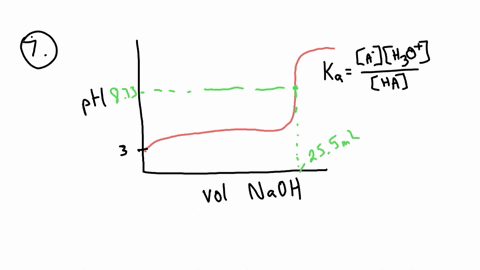
ApH/AVol NaOH IONIZATION CONSTANT OF A WEAK ACID A student performed a titration procedure to determine the Ka of an unknown acid. She then plotted her titration data to generate her titration curve. Then she performed a first derivative analysis of her titration data. Use both plots to determine the Ka of her unknown acid. 14.00 12.00 10.00 8.00 ? 6.00 0.8 0.6 0 0.2 2.00 Titration plot 0.00 0,00 10.00 20.00 30.00 40.00 50.00 60.00 70.00 Vol. NaOH (mL) First derivative 14 1.2 Indicate the following on the original titration plot... - equivalence point (eq. pt.) - volume to reach eq. pt. - volume to reach half-eq. pt. - half-eq. pt. pKa: Ka: 10 20 30 40 60 70 AVol. NaOH
ApH/AVol NaOH
IONIZATION CONSTANT OF A WEAK ACID
A student performed a titration procedure to determine the Ka of an unknown acid. She
then plotted her titration data to generate her titration curve. Then she performed a first
derivative analysis of her titration data. Use both plots to determine the Ka of her unknown
acid.
14.00
12.00
10.00
8.00
?
6.00
0.8
0.6
0
0.2
2.00
Titration plot
0.00
0,00
10.00
20.00
30.00
40.00
50.00
60.00
70.00
Vol. NaOH (mL)
First derivative
14
1.2
Indicate the following on the
original titration plot...
- equivalence point (eq. pt.)
- volume to reach eq. pt.
- volume to reach half-eq. pt.
- half-eq. pt.
pKa:
Ka:
10
20
30
40
60
70
AVol. NaOH
Show more…
Added by Francisco Javier Y.
Instant Answer
Step 1
- The plot starts at pH 0 and gradually increases as more NaOH is added. - At the equivalence point, the pH is at its highest value, indicating that the acid has been completely neutralized. - The plot then continues to increase slightly after the equivalence Show more…
Show all steps