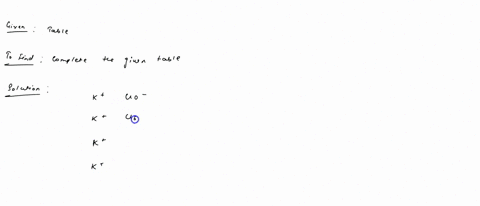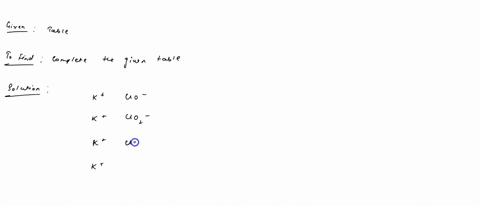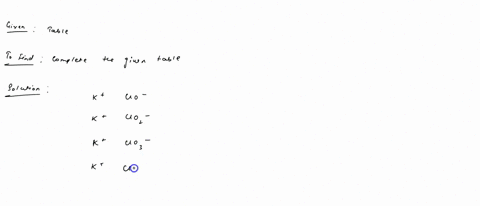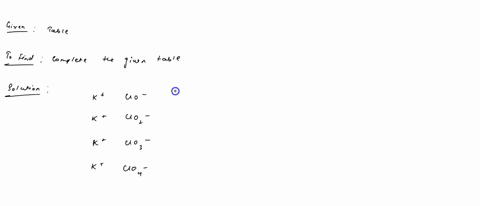Question
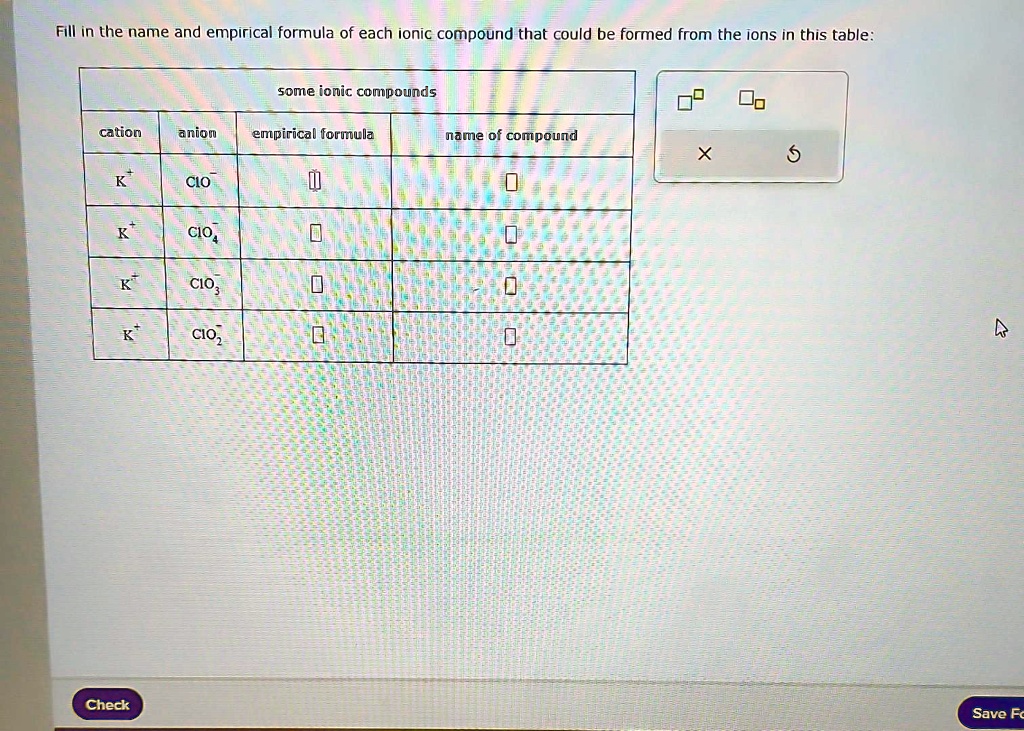
Fill in the name and empirical formula of each ionic compound that could be formed from the ions in this table: some ionic compounds cation anion empirical formula name of compound K$^+ ClO$^-$ K$^+ ClO$_4$$^-$ K$^+ ClO$_3$$^-$ K$^+ ClO$_2$$^-$ Check
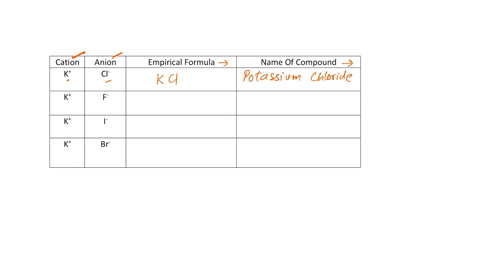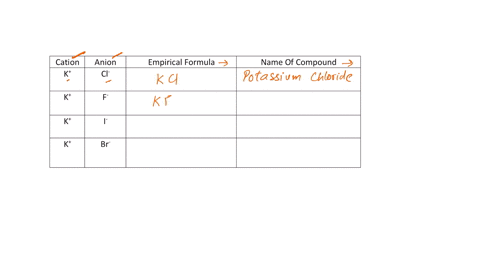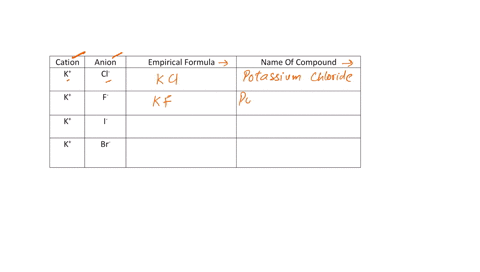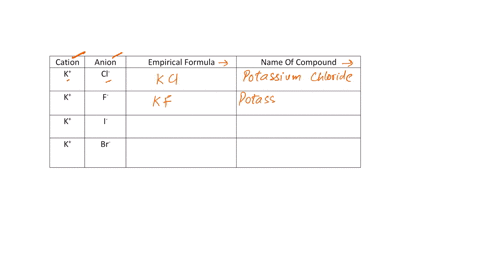
Fill in the name and empirical formula of each ionic compound that could be formed from the ions in this table:
some ionic compounds
cation
anion
empirical formula
name of compound
K$^+
ClO$^-$
K$^+
ClO$_4$$^-$
K$^+
ClO$_3$$^-$
K$^+
ClO$_2$$^-$
Check
Show more…
Added by Gloria L.
Instant Answer
Step 1
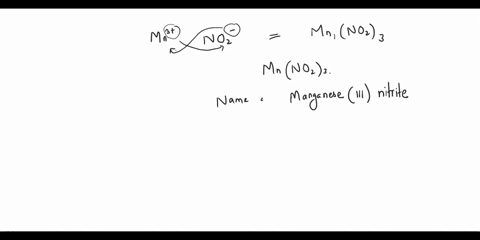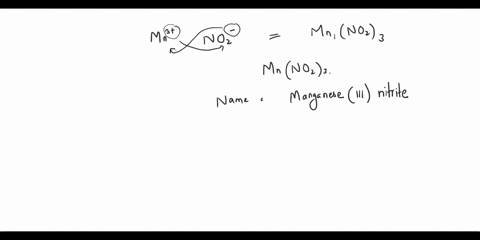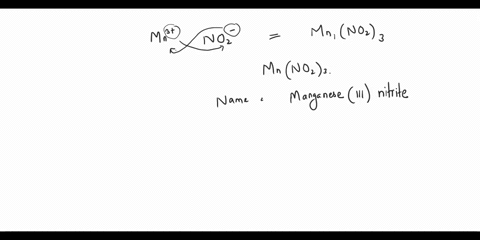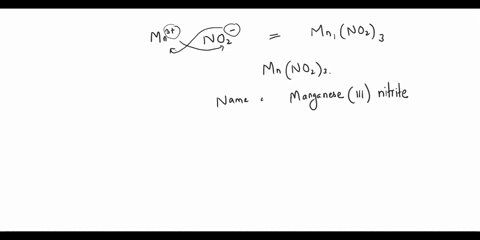
Step 2: The empirical formula for the first compound is KCl, which means it contains one potassium ion (K+) and one chloride ion (Cl-). Therefore, the name of the compound is potassium chloride. Step 3: The empirical formula for the second compound is KClO4, Show more…
Show all steps