Question
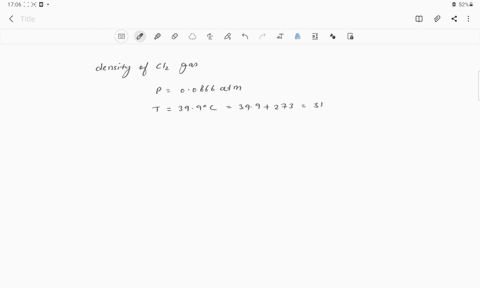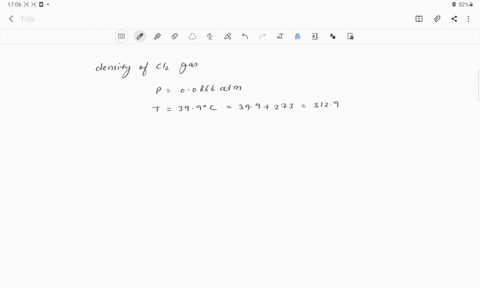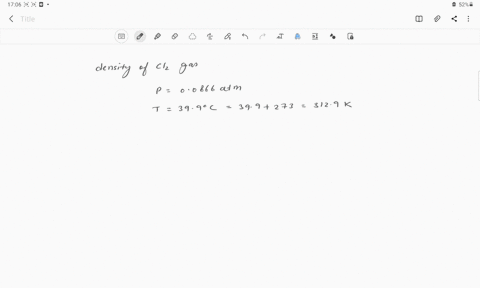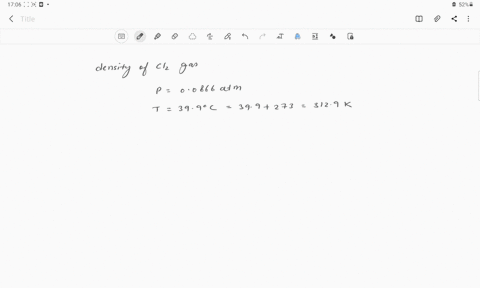
The density of chlorine gas at STP, in grams per liter, is approximately: 6.2 none of the given 3.2 0 3.9
The density of chlorine gas at STP, in grams per liter, is approximately: 6.2
none of the given 3.2
0 3.9

Added by Robert T.
Instant Answer
Step 1
9 g/mol. Second, we know that at STP (Standard Temperature and Pressure), one mole of any gas occupies a volume of 22.4 liters. Show more…
Show all steps