Question
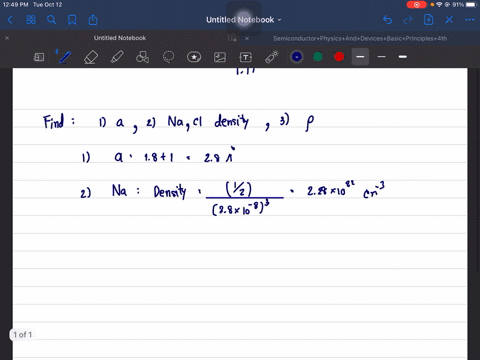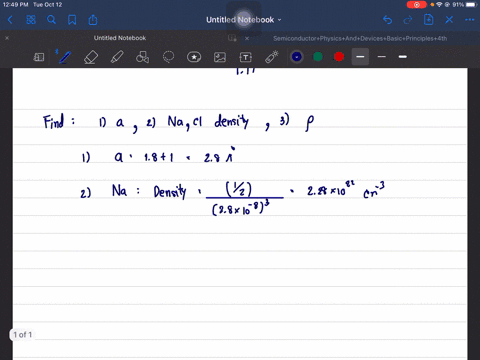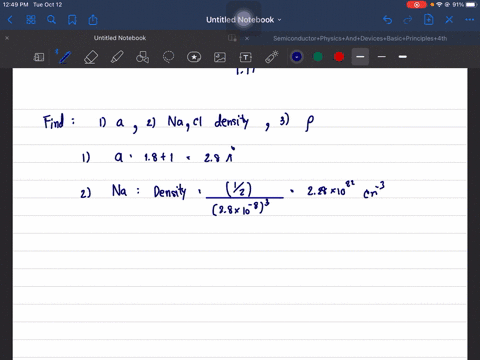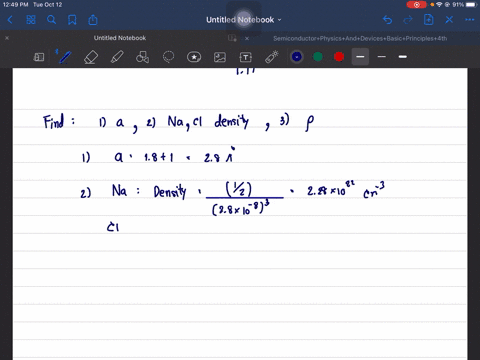
The structure of NaCl is given. Determine its lattice constant, a, and its density. Hint: since NaCl is ionic use the ion radius data and the atomic radii.
The structure of NaCl is given. Determine its lattice constant,
a, and its density. Hint: since NaCl is ionic use the ion radius
data and the atomic radii.

Added by Leslie P.
Instant Answer
Step 1
Determine the ion radii of Na+ and Cl- ions. The ionic radius of Na+ is 0.95 Å (angstroms). The ionic radius of Cl- is 1.81 Å (angstroms). Show more…
Show all steps