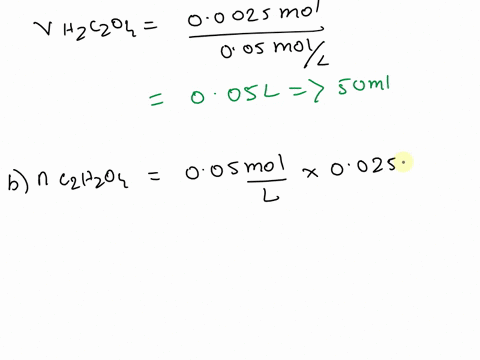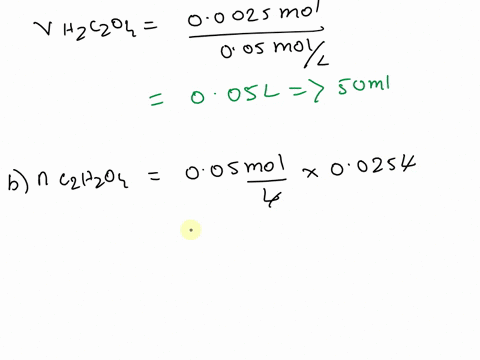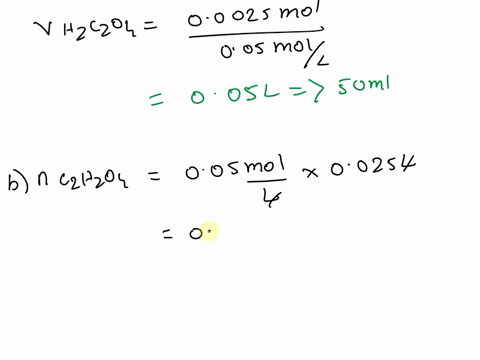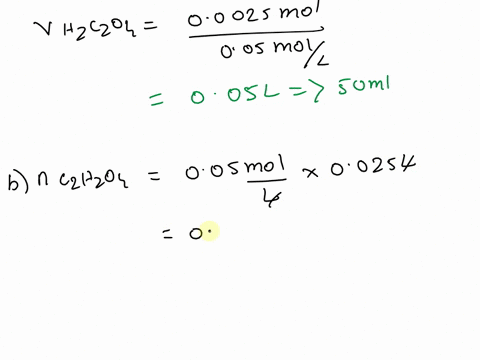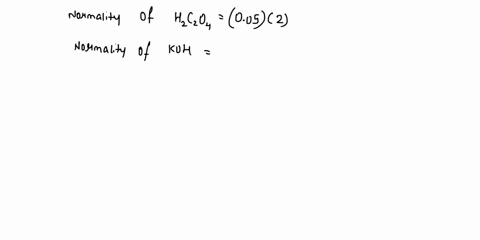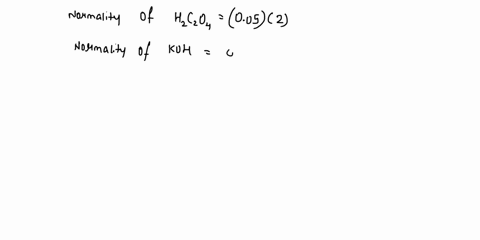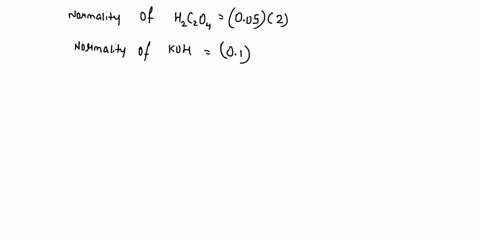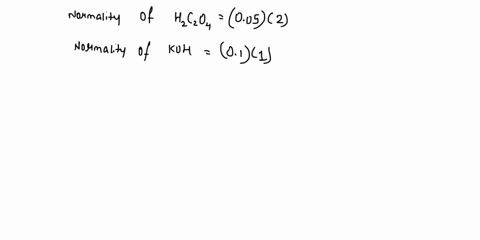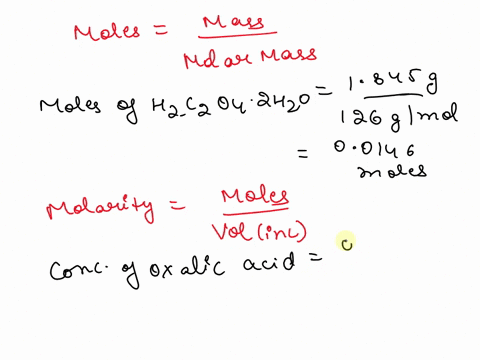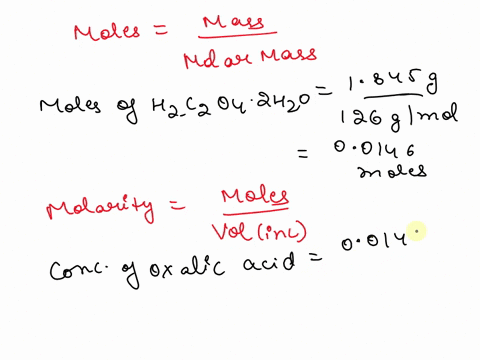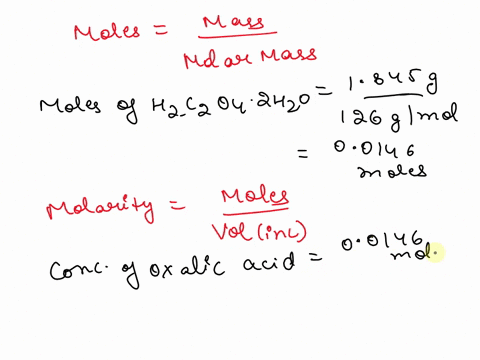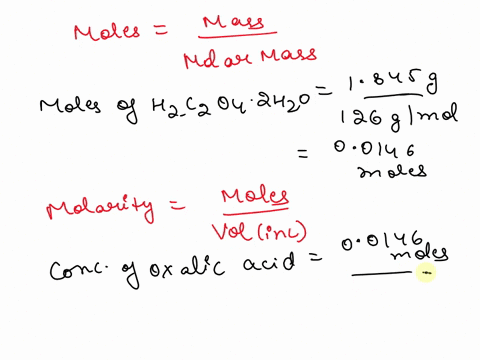Question
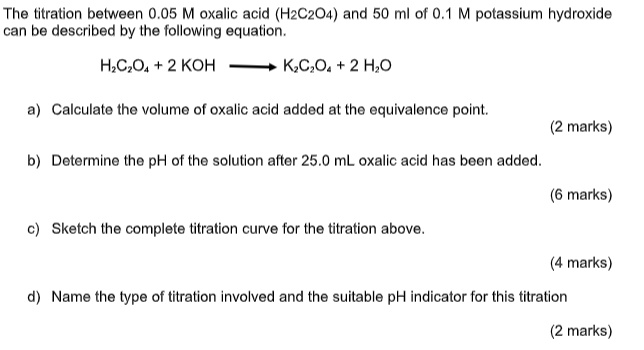
The titration between 0.05 M oxalic acid ($H_2C_2O_4$) and 50 ml of 0.1 M potassium hydroxide can be described by the following equation. $H_2C_2O_4 + 2 KOH \longrightarrow K_2C_2O_4 + 2 H_2O$ a) Calculate the volume of oxalic acid added at the equivalence point. (2 marks) b) Determine the pH of the solution after 25.0 mL oxalic acid has been added. (6 marks) c) Sketch the complete titration curve for the titration above. (4 marks) d) Name the type of titration involved and the suitable pH indicator for this titration (2 marks)
The titration between 0.05 M oxalic acid ($H_2C_2O_4$) and 50 ml of 0.1 M potassium hydroxide can be described by the following equation.
$H_2C_2O_4 + 2 KOH \longrightarrow K_2C_2O_4 + 2 H_2O$
a) Calculate the volume of oxalic acid added at the equivalence point.
(2 marks)
b) Determine the pH of the solution after 25.0 mL oxalic acid has been added.
(6 marks)
c) Sketch the complete titration curve for the titration above.
(4 marks)
d) Name the type of titration involved and the suitable pH indicator for this titration
(2 marks)
Show more…
Added by Aaron G.
Instant Answer
Step 1
The stoichiometry of the reaction is 1:2, meaning one mole of oxalic acid reacts with two moles of potassium hydroxide. Show more…
Show all steps