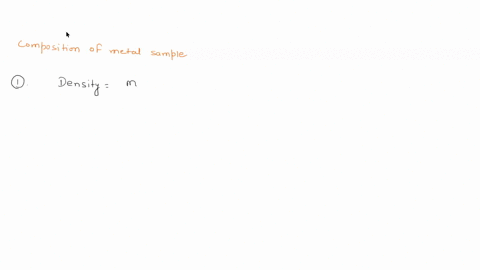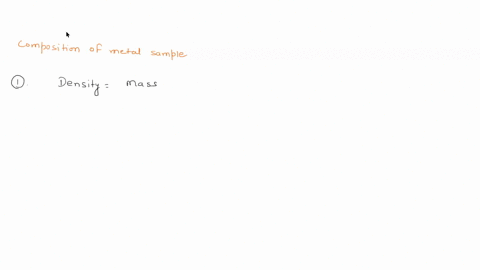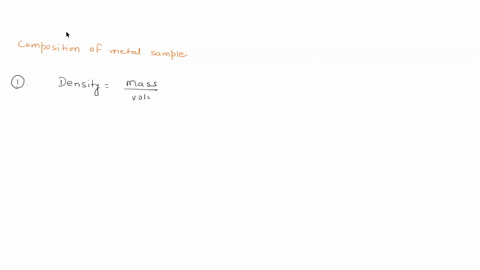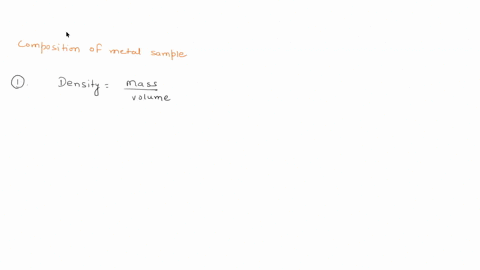Question
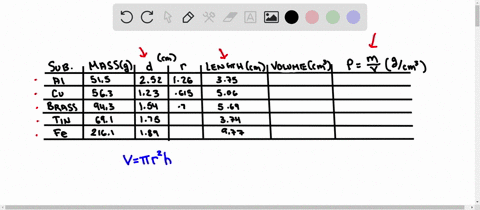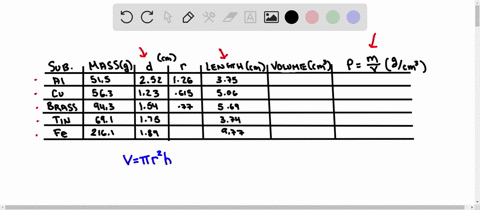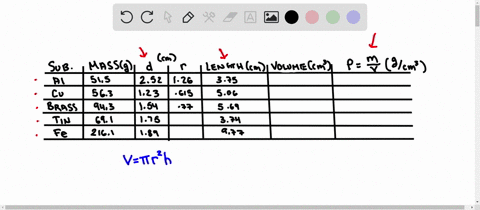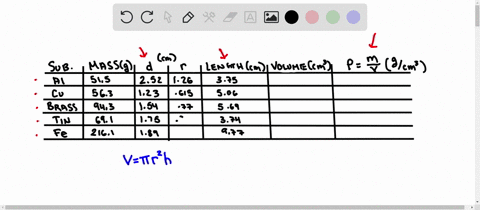
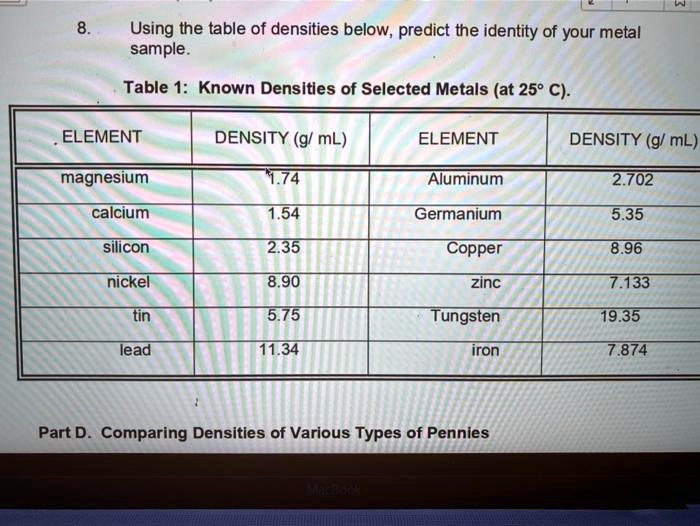
Using the table of densities below, predict the identity of your metal sample Table 1: Known Densities of Selected Metals (at 259 C): ELEMENT DENSITY (g/ mL) ELEMENT DENSITY (g/ mL) magnesium 1.74 Aluminum 2.702 calcium 1.54 Germanium 5.35 silicon 235 Copper 8.96 nickel 8.90 zinc 7.733 tin 5.75 Tungsten 79.35 lead 11.34 iron 7.874 Part D Comparing Densities of Various Types of Pennies
Using the table of densities below, predict the identity of your metal sample
Table 1: Known Densities of Selected Metals (at 259 C):
ELEMENT
DENSITY (g/ mL)
ELEMENT
DENSITY (g/ mL)
magnesium
1.74
Aluminum
2.702
calcium
1.54
Germanium
5.35
silicon
235
Copper
8.96
nickel
8.90
zinc
7.733
tin
5.75
Tungsten
79.35
lead
11.34
iron
7.874
Part D Comparing Densities of Various Types of Pennies
Show more…
Added by Shari F.
Instant Answer
Step 1
To do this, we need to know the mass and volume of the sample. Unfortunately, you have not provided this information. Assuming you have the mass and volume of the metal sample, you can calculate its density using the formula: Density = $\frac{Mass}{Volume}$ Show more…
Show all steps