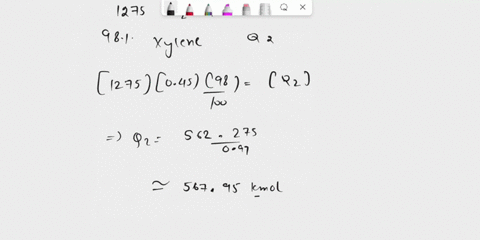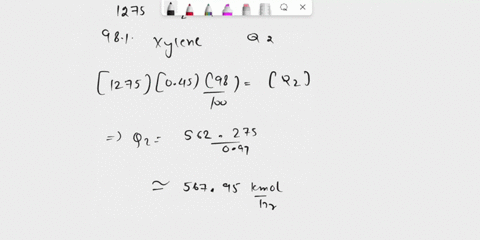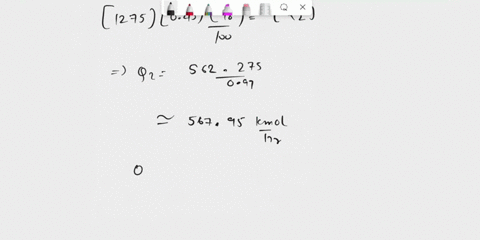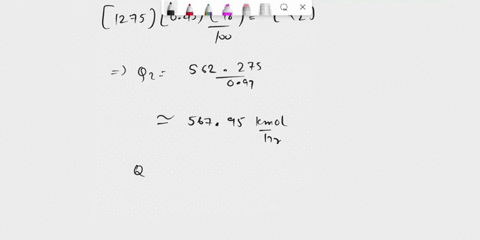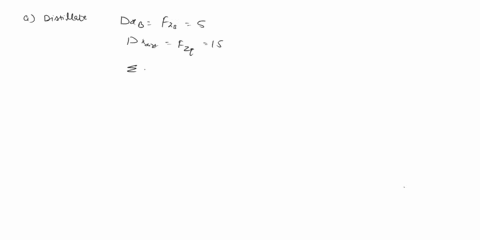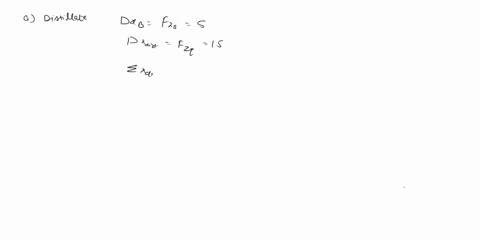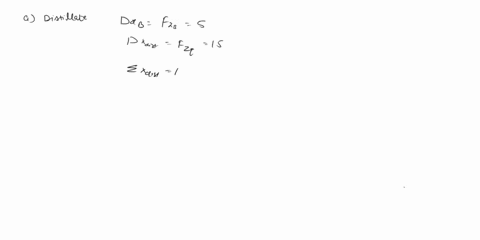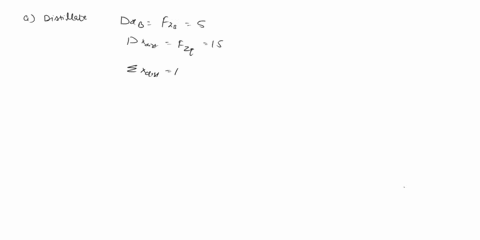Question
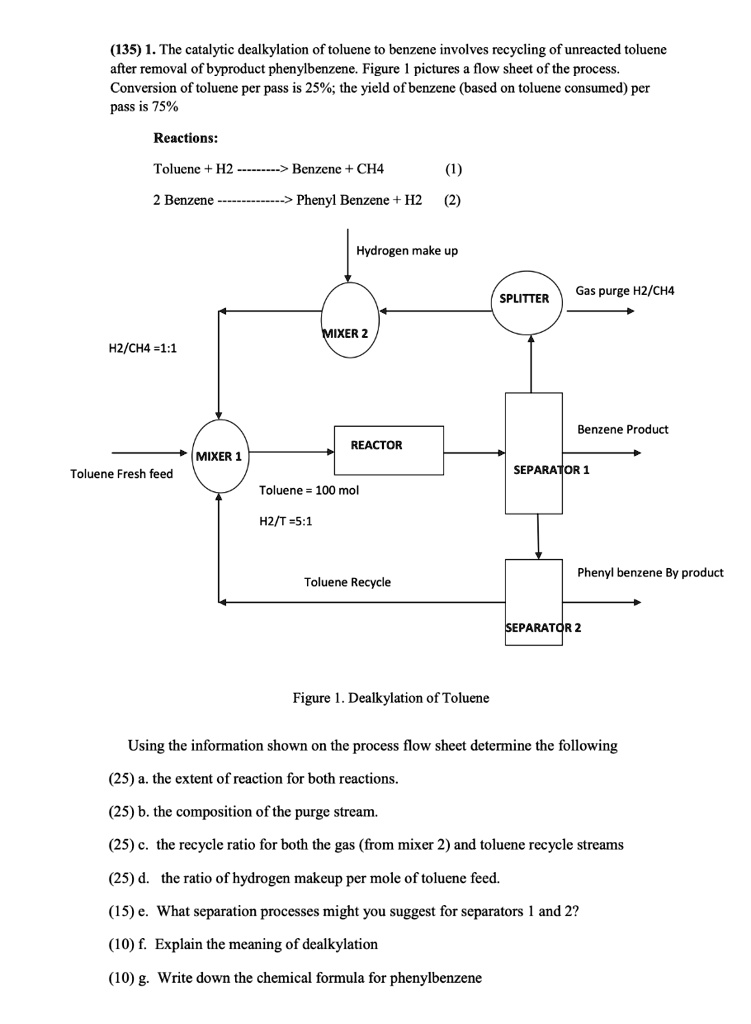
(135) 1. The catalytic dealkylation of toluene to benzene involves recycling of unreacted toluene after removal of byproduct phenylbenzene. Figure 1 pictures a flow sheet of the process. Conversion of toluene per pass is 25%; the yield of benzene (based on toluene consumed) per pass is 75% Reactions: Toluene + H2 $\rightarrow$ Benzene + CH4 (1) 2 Benzene $\rightarrow$ Phenyl Benzene + H2 (2) Hydrogen make up H2/CH4 =1:1 Toluene Fresh feed Toluene = 100 mol H2/T =5:1 Toluene Recycle Using the information shown on the process flow sheet determine the following (25) a. the extent of reaction for both reactions. (25) b. the composition of the purge stream. (25) c. the recycle ratio for both the gas (from mixer 2) and toluene recycle streams (25) d. the ratio of hydrogen makeup per mole of toluene feed. (15) e. What separation processes might you suggest for separators 1 and 2? (10) f. Explain the meaning of dealkylation (10) g. Write down the chemical formula for phenylbenzene
(135) 1. The catalytic dealkylation of toluene to benzene involves recycling of unreacted toluene
after removal of byproduct phenylbenzene. Figure 1 pictures a flow sheet of the process.
Conversion of toluene per pass is 25%; the yield of benzene (based on toluene consumed) per
pass is 75%
Reactions:
Toluene + H2 $\rightarrow$ Benzene + CH4
(1)
2 Benzene $\rightarrow$ Phenyl Benzene + H2
(2)
Hydrogen make up
H2/CH4 =1:1
Toluene Fresh feed
Toluene = 100 mol
H2/T =5:1
Toluene Recycle
Using the information shown on the process flow sheet determine the following
(25) a. the extent of reaction for both reactions.
(25) b. the composition of the purge stream.
(25) c. the recycle ratio for both the gas (from mixer 2) and toluene recycle streams
(25) d. the ratio of hydrogen makeup per mole of toluene feed.
(15) e. What separation processes might you suggest for separators 1 and 2?
(10) f. Explain the meaning of dealkylation
(10) g. Write down the chemical formula for phenylbenzene
Show more…
Added by Patricia Y.
Instant Answer
Step 1
The extent of reaction for reaction (1) can be calculated using the conversion of toluene per pass, which is 25%. The extent of reaction is defined as the ratio of the moles reacted to the moles fed. Since the stoichiometric ratio between toluene and benzene in Show more…
Show all steps