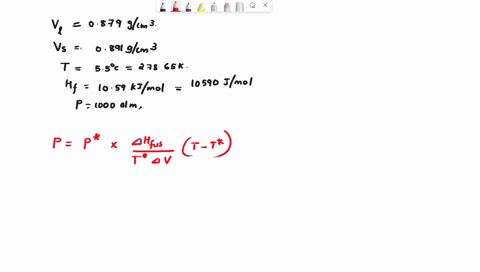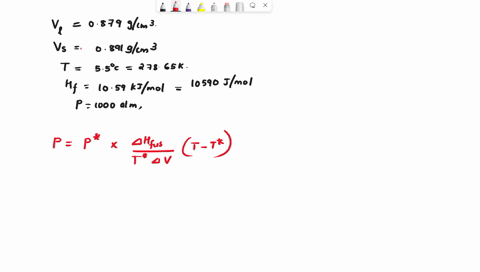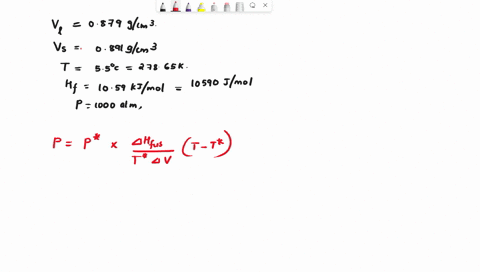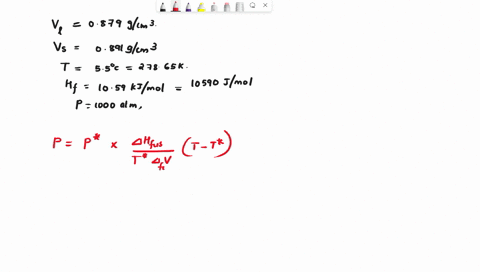Question
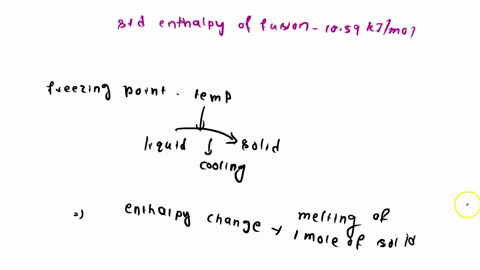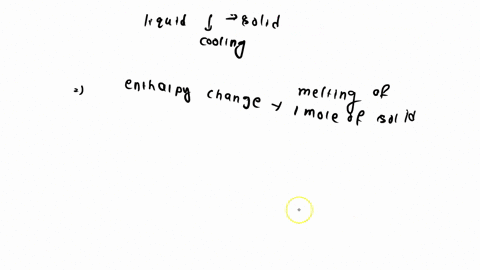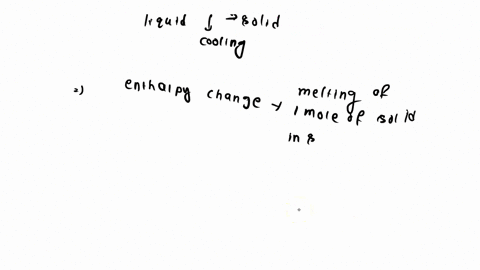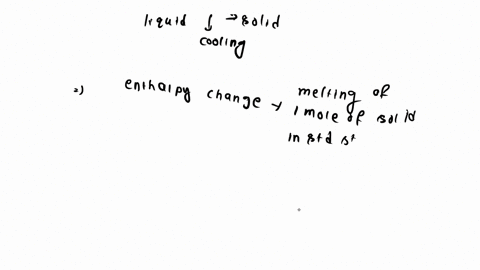
When benzene freezes at 5.5 "C its density changes from 0.879 g cm to 0.891 g cm Its enthalpy of fusion is 10.59 kJ mol Estimate the freezing point of benzene at [000 atm
When benzene freezes at 5.5 "C its density changes from 0.879 g cm to 0.891 g cm Its enthalpy of fusion is 10.59 kJ mol Estimate the freezing point of benzene at [000 atm

Added by Sean D.
Instant Answer
Step 1
First, we need to find the molar volume change during the phase transition (from liquid to solid). We can do this by dividing the molar mass of benzene by its density in each phase: Molar mass of benzene (C6H6) = 6 * 12.01 (C) + 6 * 1.01 (H) = 78.12 g/mol Molar Show more…
Show all steps