Question
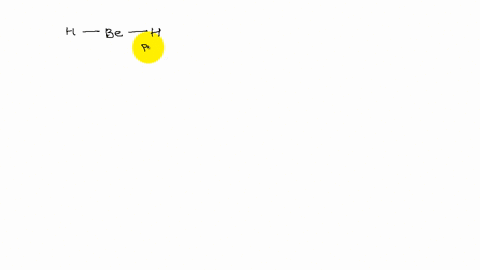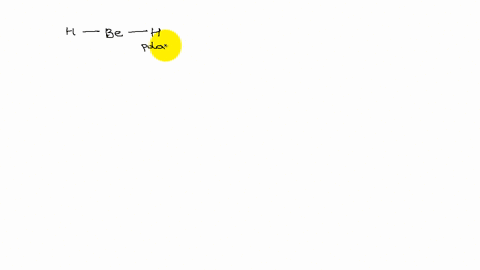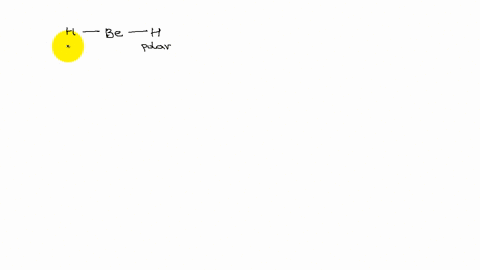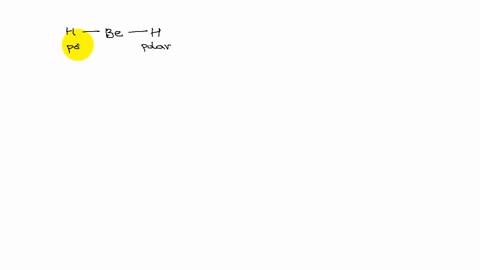
XeF4 and XeF2 have lone pairs of electrons on the central atom that is non polar. Explain why they have no dipole moment.
XeF4 and XeF2 have lone pairs of electrons on the central atom
that is non polar. Explain why they have no dipole
moment.
Added by Ruben H.
Instant Answer
Step 1
- XeF4 (Xenon Tetrafluoride) has a square planar geometry. - XeF2 (Xenon Difluoride) has a linear geometry. Show more…
Show all steps