Question
How do you draw an ionic Lewis structure?
How do you draw an ionic Lewis structure?
Chapter 9, Problem 7

Instant Answer
Step 1
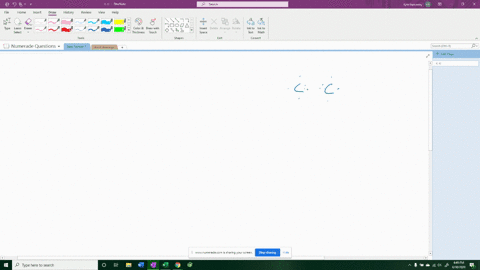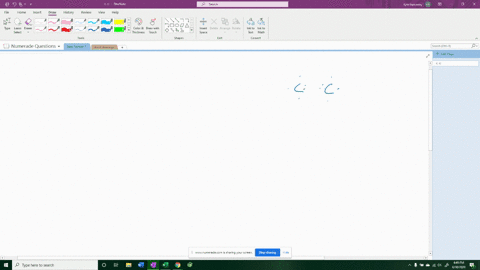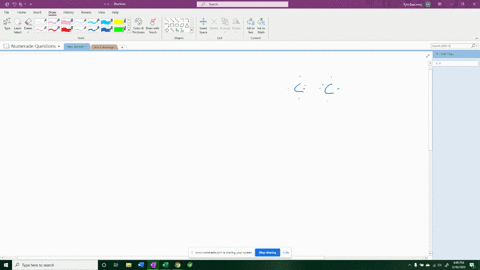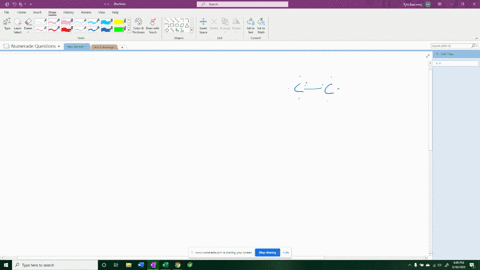
For example, in potassium iodide, potassium (K) has one valence electron and iodine (I) has seven valence electrons. Show more…
Show all steps