Question
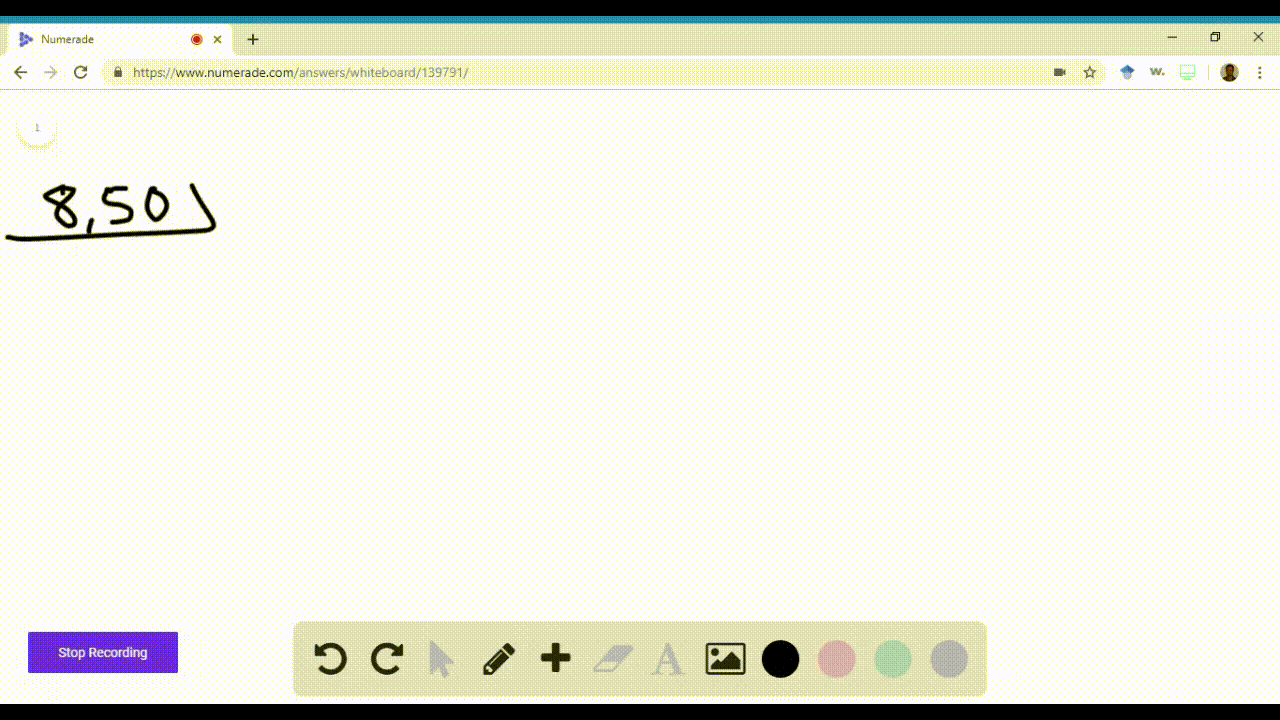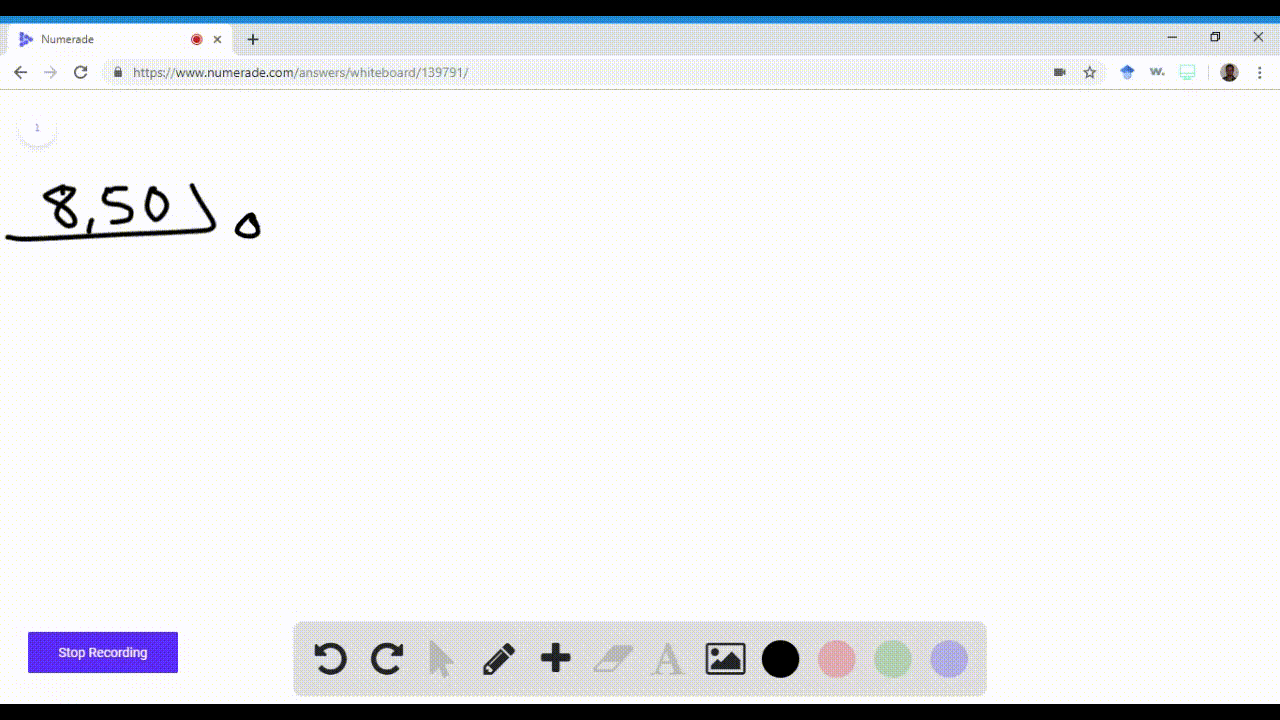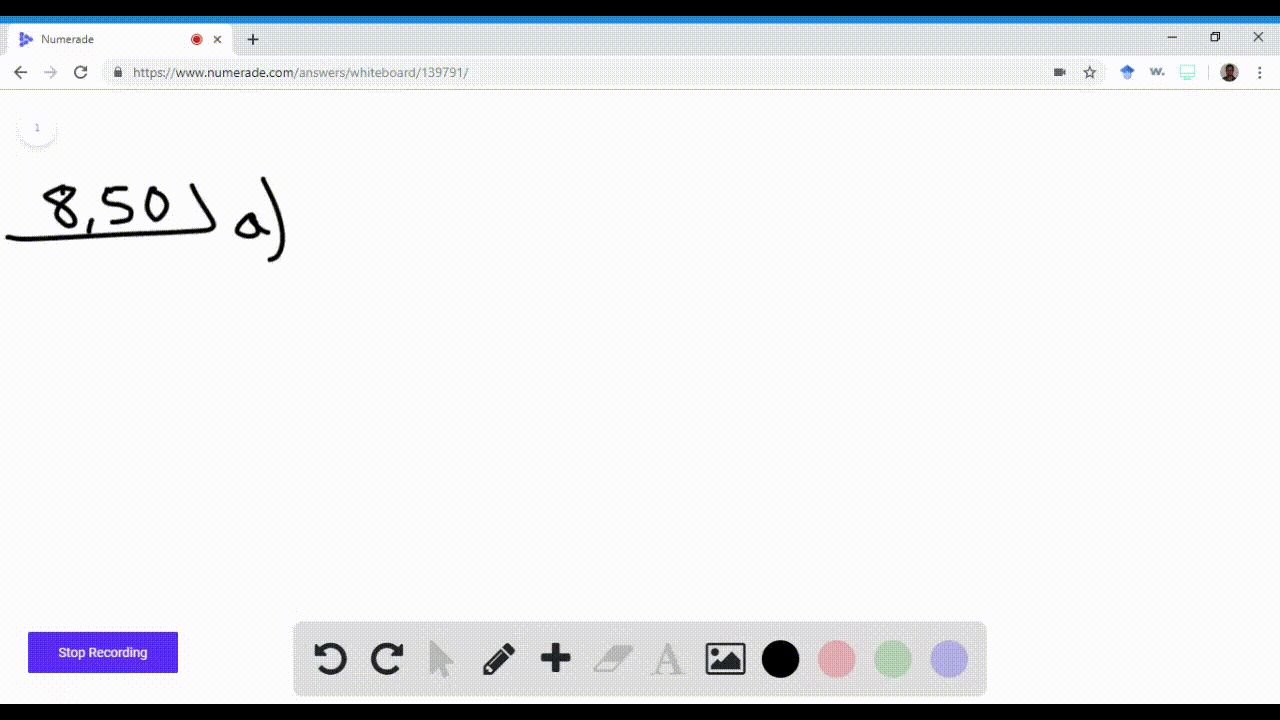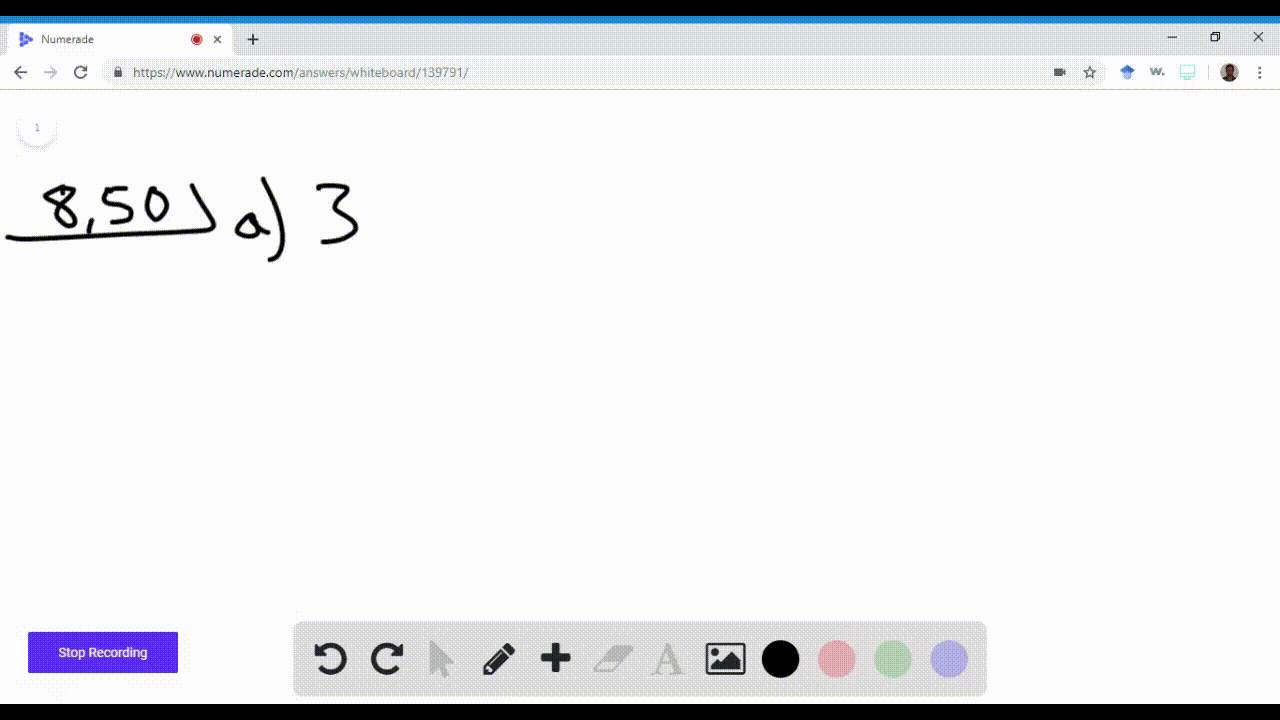
Name an element in the fourth period (row) of the periodic table with the following: \begin{equation} \begin{array}{l}{\text { a. five valence electrons }} \\ {\text { b. four 4p electrons }}\end{array} \end{equation}\begin{equation} \begin{array}{l}{\text { c. three } 3 d \text { electrons }} \\ {\text { d. full s and p sublevels }}\end{array} \end{equation}
Name an element in the fourth period (row) of the periodic table with the following:Show more…
\begin{equation}
\begin{array}{l}{\text { a. five valence electrons }} \\ {\text { b. four 4p electrons }}\end{array}
\end{equation}\begin{equation}
\begin{array}{l}{\text { c. three } 3 d \text { electrons }} \\ {\text { d. full s and p sublevels }}\end{array}
\end{equation}
Chapter 8, Problem 49

Instant Answer
Step 1
Valence electrons are the electrons in the outermost shell of an atom. In the fourth period, the elements transition from the 4s to the 3d and then to the 4p subshells. If we consider the 4s and 3d as the highest energy subshells, then Vanadium (V) has five Show more…
Show all steps