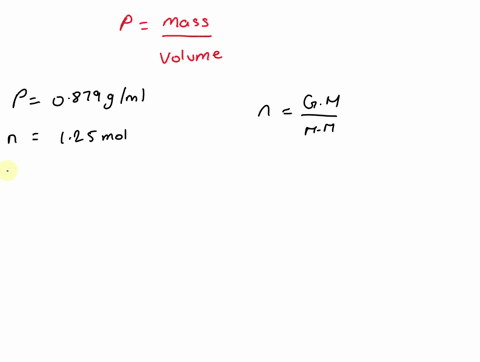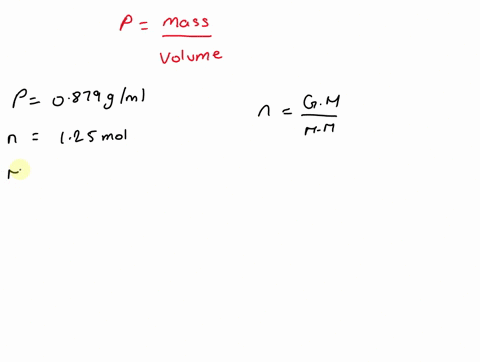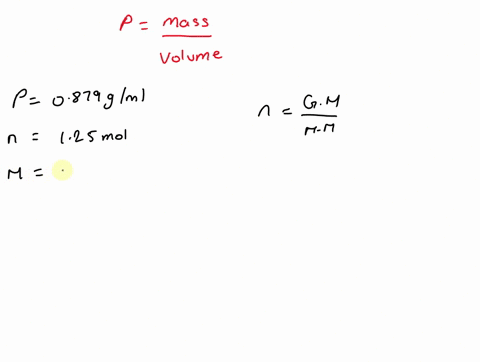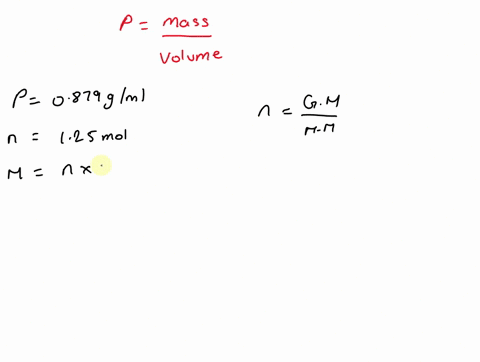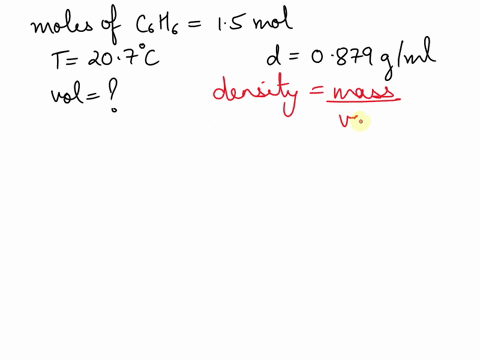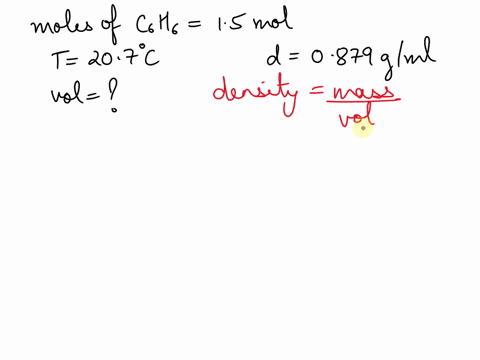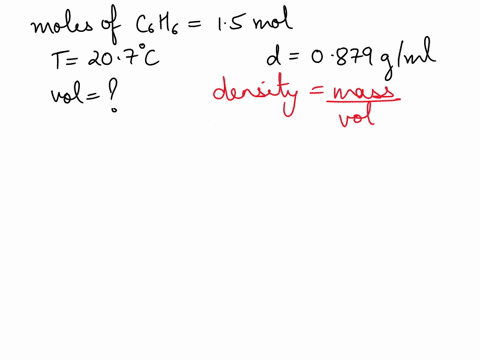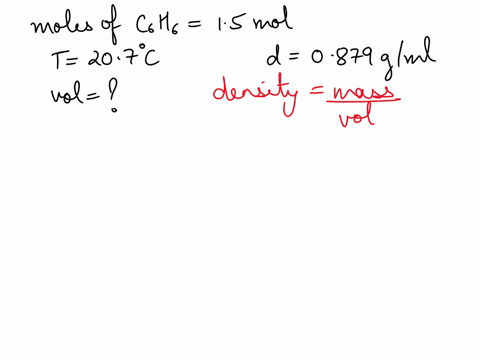Question
What is the volume of 1.00 mol benzene $\left(\mathrm{C}_{6} \mathrm{H}_{6}\right)$ at $20^{\circ} \mathrm{C} ?$ The density of benzene is $0.879 \mathrm{g} / \mathrm{mL}$.
What is the volume of 1.00 mol benzene $\left(\mathrm{C}_{6} \mathrm{H}_{6}\right)$ at $20^{\circ} \mathrm{C} ?$ The density of benzene is $0.879 \mathrm{g} / \mathrm{mL}$.
Chapter 3, Problem 42

Instant Answer
Step 1
The molar mass of carbon (C) is approximately 12.01 g/mol and the molar mass of hydrogen (H) is approximately 1.01 g/mol. Therefore, the molar mass of benzene is: \[6(12.01 \, \text{g/mol}) + 6(1.01 \, \text{g/mol}) = 78.12 \, \text{g/mol}\] Show more…
Show all steps